Force stabilises a bond in bacterial adhesion
October 25, 2022NUS biophysicists have discovered how a special bacterial adhesion complex could be counterintuitively stabilised by mechanical stress at the single-molecule level.
A critical aspect of bacterium-host interactions is the physical contact between bacteria and hosts as well as their responses to local mechanical stimuli. The bacterial adhesion complexes formed with the hosts need to be resilient to various mechanical stress conditions, associated with forces over a wide range from nanonewtons in the urinary tract down to piconewtons in the capillaries. On the other hand, the adhered bacteria need to retain the ability to spread, demanding sufficiently short lifetimes of the adhesion complexes at stress-free conditions. A long-standing hypothesis is that bacterial adhesion complexes may have evolved with a unique mechanical property, conferring them a counter-intuitive force-dependent increase of lifetime, which is known as catch-bond kinetics. To some extent, this is analogous to the safety belts found in cars, which tighten up when there is a sudden increase in stresses. The test of this important hypothesis has been hindered for years by a technological challenge of being able to quantify the force-dependent lifetimes of a single bacterial adhesion complex over a force range of up to tens of piconewtons for long durations.
A research team led by Professor Jie YAN from the Department of Physics, National University of Singapore has overcome the technological challenge and investigated the force-dependent lifetimes of a family of bacterial adhesion complexes using ultra-stable magnetic-tweezers. Strong catch-bond kinetics are observed for all these adhesion complexes, with the lifetimes increased by several thousands of folds when the applied forces increase from near-zero to tens of piconewtons. A highly sensitive temperature dependence of the lifetimes is also revealed. This is shown by a drastic decrease in the lifetime of more than 100 folds when the temperature increases from 23oC to the human body temperature of 37oC.
Prof Yan said, “A unique force-dependent conformational change of the adhesion complexes is found to be the rupturing transition pathway of the adhesion complexes. With this, the team derived a physical model that can quantitatively explain the observed catch-bond force-dependent lifetimes of the adhesion complexes, which is the first ever reported proof of this catch-bond kinetics in bacteria.”
In future, the research team will extend their technology to examine the mechanical stability of a broader range of microbial adhesion complexes implicated in human diseases and explore possible intervening approaches to destabilise the bacterial adhesions.
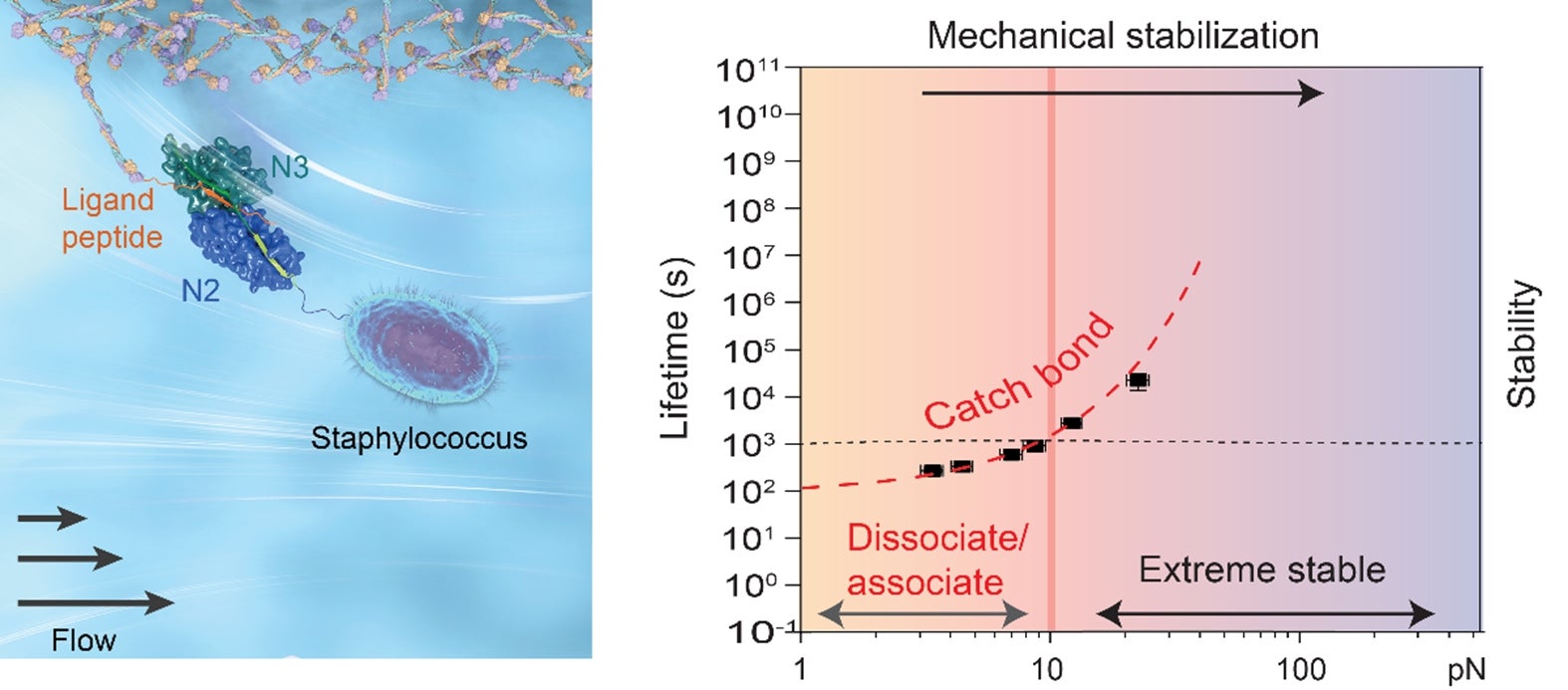
Illustration shows a staphylococcus bacterium adhered to human host ligand through the typical bacterial adhesion protein (left), and the counterintuitively catch-bond behaviour with increased lifetime under increasing forces. [Credit: Journal of the American Chemical Society]
Reference
Huang W; Le S; SunY; Lin DJ; Yao M; Shi Y; Yan J*, “Mechanical Stabilization of a Bacterial Adhesion Complex” JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 144 Issue: 37 Page: 16808–16818 DOI: 10.1021/jacs.2c03961 Published: 2022.


