Sustainable electro-synthesis of esters
July 27, 2022NUS chemists have discovered how acetate esters could be electro-synthesised from water and carbon monoxide in an environmentally sustainable way.
The electrocatalytic reduction of carbon dioxide or carbon monoxide using renewable electricity is a promising green manufacturing method for the production of chemicals. Thus far, a wide range of carbonaceous products including alcohols, hydrocarbons and carboxylic acids has been manufactured using this method. Interestingly, esters, an important family of organic compounds, have not been formed in the same way. There are many uses for esters, some of which include solvents and lubricants. They are classically produced through stoichiometric reactions such as Fischer esterification. The starting reagents are typically derived from fossil resources, and chemical waste is generated after the reaction, which renders the process environmentally unfriendly.
A research team led by Associate Professor YEO Boon Siang, Jason from the Department of Chemistry, National University of Singapore in collaboration with Dr Sumit VERMA and Dr Ramesha GANGANAHALLI from the international energy company Shell has discovered how C3 – C6 acetate esters can be electro-synthesised from carbon monoxide and water using copper catalysts in a membrane electrode assembly. Ethyl acetate and propyl acetate can be produced with a total Faradaic efficiency (FE) of about 22% and with a current density of up to 55 mA/cm2, alongside minor quantities of methyl acetate and butyl acetate. The esters were produced via the addition reaction of ethenone (H2C=C=O) and alcohols produced during CO reduction (see Figure). The near water-free reaction condition and the high local pH level play key roles in the formation of the esters.
NUS and Shell had agreed earlier last year to jointly develop processes to create green fuels and chemicals for the energy industry (see link).
Prof Yeo said, “This work is a result of the collaboration between NUS and Shell to sustainably create chemicals. Our team has succeeded in developing an electrochemically-driven process to make esters using renewable feedstocks such as carbon monoxide and water.”
Building on the research findings from their work, the research team plans to develop catalysts with higher ester conversion efficiency.
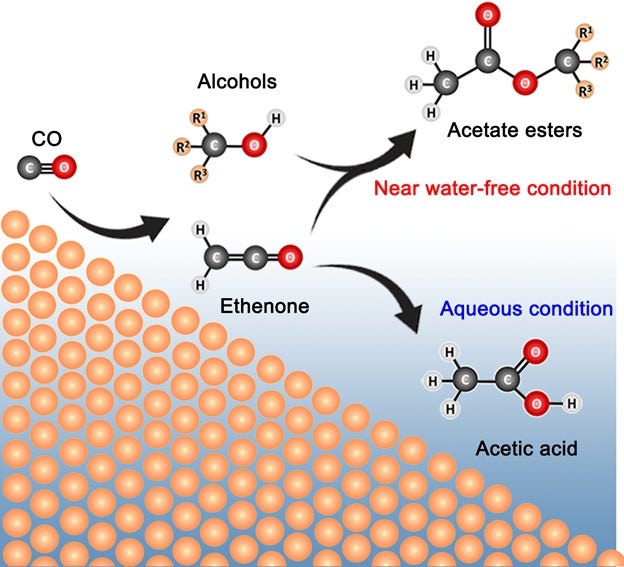
Visual shows the processes involved in the production of C3 – C6 acetate esters from the electrocatalytic reduction of carbon monoxide in a membrane electrode assembly cell. [Credit: Angewandte Chemie International Edition]
Reference
Zhou, R. Ganganahalli, S. Verma, H.R. Tan, B.S. Yeo*, “Production of C3 – C6 Acetate Esters via CO Electroreduction in a Membrane Electrode Assembly Cell” ANGEWANDTE CHEMIE INTERNATIONAL EDITION DOI: 10.1002/anie.202202859 Published: 2022.


