Efficacious and safe drug for the treatment of atrial fibrillation
July 18, 2022NUS pharmaceutical scientists have developed an improved pharmaceutical drug for the treatment of irregular heart rhythm, known as atrial fibrillation.
Irregular rhythm in the upper heart chambers (atrial fibrillation) is a serious condition that can lead to cardiac arrest or stroke. For patients suffering from atrial fibrillation, there are pharmaceutical drugs which help manage and provide them with a typical heart rhythm. Ironically, these drugs promote irregular heart rhythm in the lower heart chambers (ventricular arrhythmia) which is potentially dangerous or even fatal. Some of these medications may also have other potential side effects that cause organ failures. Hence, there is an impetus to develop efficacious and safer pharmaceutical drugs to treat atrial fibrillation.
A research team led by Professor Eric CHAN from the Department of Pharmacy, National University of Singapore has discovered that these drugs cause the inactivation of a key heart enzyme and in turn this causes ventricular arrhythmia. Armed with this knowledge, they successfully circumvented this adverse effect by modifying the chemical structure of a clinically prescribed drug. This is achieved through the subtle replacement of selected hydrogen atoms present on the drug molecule with deuterium atoms, in a process known as deuteration. Through this change, the drug molecule is no longer able to inactivate the key heart enzyme. The team performed additional tests and further determined that the improved drug retained several favourable drug-related properties (for example, the pharmacokinetic and pharmacology profile). The development of an improved version of an existing drug that is both efficacious and safer is important to address the unmet medical need in atrial fibrillation therapy.
The predecessor drug, dronedarone, is metabolised by cytochrome P450 2J2 or CYP2J2, a highly expressed enzyme in the heart, into an electron-deficient or electrophilic metabolite that can irreversibly bind to the active site of CYP2J2, inactivating it. The researchers elucidated the chemical structure of the electrophilic metabolite and reduced its chemical reactivity by exchanging the hydrogen atoms with its heavier isotopic atom, deuterium. By changing the carbon-deuterium bond strength in this new drug termed as poyendarone, the reactivity of the metabolite is reduced. This curtailed the inactivation of CYP2J2 and the adverse effects associated with it. Using canine models, poyendarone is shown to be effective in treating atrial fibrillation and it does not demonstrate the adverse effects observed for dronedarone. The team reported that this is the first time deuteration as a drug discovery strategy is applied to optimise the pharmacology while mitigating the toxicology of a drug molecule.
With poyendarone being a patented drug asset, the team is liaising with an industry partner on the use of this drug for clinical investigation as part of its developmental process.
Prof Chan said, “Our research findings demonstrate an innovative discovery platform that can enhance patient care via the development of efficacious and safe pharmaceuticals.”
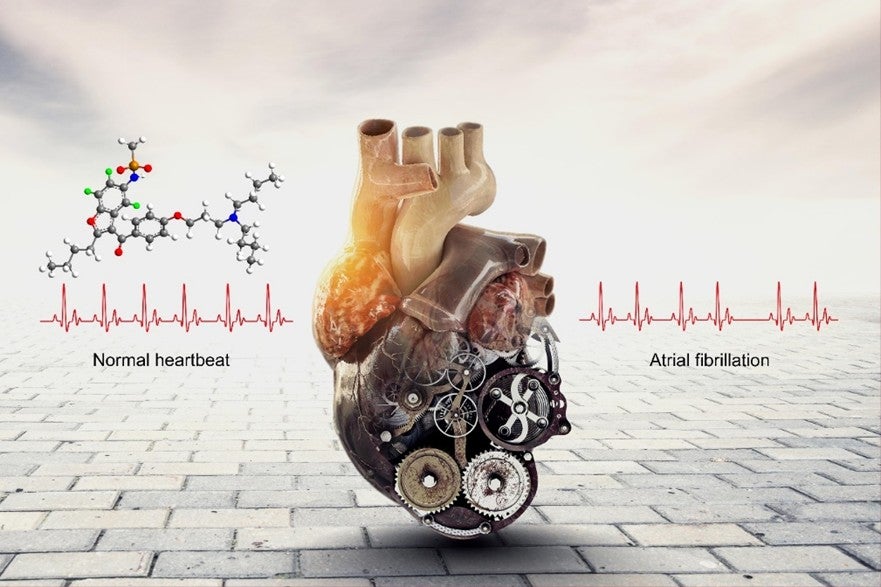
Illustration showing that poyendarone is an efficacious and safe drug for treatment of atrial fibrillation. For the molecular structure, the green balls represent selected sites in which the hydrogen atoms had been replaced with deuterium atoms. The white balls represent sites containing the remaining hydrogen atoms. These seemingly “subtle” chemical modifications change the properties of the drug significantly in positive ways.
Reference
Karkhanis A; Venkatesan G; Kambayashi R; Leow JWH; Han MQ; Izumi-Nakaseko H; Goto A; Pang JKS; Soh BS; Kojodjojo P; Sugiyama A; Chan ECY*, “Site-directed deuteration of dronedarone preserves cytochrome P4502J2 activity and mitigates its cardiac adverse effects in canine arrhythmic hearts” ACTA PHARMACEUTICA SINICA B DOI: 10.1016/j.apsb.2022.03.008 In Press.


