A biologic drug for the treatment of chronic obstructive pulmonary disease
April 24, 2022NUS scientists have discovered a novel anti-inflammatory action of a lung resident protein Isthmin 1 (ISM1) and demonstrated its potential as a biologic drug for the treatment of chronic obstructive pulmonary disease (COPD).
COPD is currently the third-leading cause of death globally and poses a large socioeconomic burden on nations. It can be caused by long-term exposure to irritants or particulate matter, such as cigarette smoke, and symptoms include coughing, breathing difficulties, mucus production and wheezing.
Patients with COPD display two key conditions – emphysema (the destruction of alveolar walls and enlargement of the alveoli) and chronic obstructive bronchitis (inflamed small airways). These patients suffer persistent respiratory symptoms with progressive long-term lung function decline. However, current drugs targeting COPD only provide symptomatic relief and are not able to suppress the underlying tissue inflammation to effectively block the spread of COPD or reduce mortality.
The ability to control inflammation is of paramount importance for lung health. A research team led by Associate Professor GE Ruowen from the Department of Biological Sciences, National University of Singapore (NUS) recently revealed that ISM1 is critical for restraining inflammation in a healthy lung by selectively eliminating the pro-inflammatory alveolar macrophages. ISM1 achieved this by targeting the GRP78 signalling receptor (csGRP78) present on the cell surface of the alveolar macrophages. Alveolar macrophages are immune cells residing in the airspace of lung breathing structures called alveoli and they can display either pro-inflammatory or anti-inflammatory properties. In COPD, the pro-inflammatory alveolar macrophages are amplified, which is a main cause of COPD.
Dr Terence LAM, a research fellow from Prof Ge’s laboratory said, “We previously identified ISM1 as an anti-angiogenic and pro-apoptotic protein that inhibited tumour growth. However, the physiological functions of ISM1 remain unclear.”
In collaboration with Professor Fred WONG of the Department of Pharmacology, NUS, the team conduct laboratory studies to examine the effectiveness of delivering recombinant ISM1 protein directly via droplets into the airway. The researchers observed a reduction in lung inflammation, suppression of emphysema development, and restoration of lung functions. These findings lend support to the development of ISM1 as a potential treatment for COPD.
Prof Ge said, “By directly targeting pro-inflammatory alveolar macrophages using recombinant ISM1 protein, our novel treatment suppresses the root cause of COPD, and opens up the possibility of developing this into a viable treatment for this debilitating disease that affects many patients around the world.”
“These findings not only provide a new avenue to develop novel and effective drugs for COPD, but also warrant studies of ISM1 in other inflammatory respiratory diseases,” added Prof Wong.
The team plans to further study the structure-activity relationship of ISM1 to facilitate drug development. They also plan to investigate whether ISM1 has a wider anti-inflammatory role in other respiratory diseases such as allergic asthma, acute lung injury and pulmonary fibrosis.
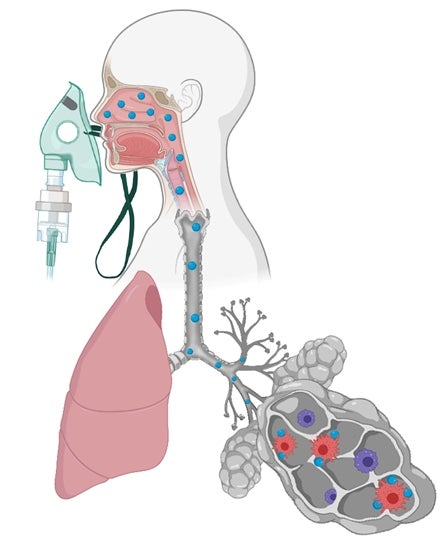 |
A schematic diagram depicting the aerosolised ISM1 biologic targeting the proinflammatory alveolar macrophages via inhalation therapy. The investigators identified ISM1 as an anti-inflammatory protein that promotes lung homeostasis by selectively eliminating proinflammatory alveolar macrophages. This approach can be exploited to develop novel protein-based therapeutics for inflammatory lung diseases. Blue particles represent ISM1 protein. |
Reference
Lam YWT; Nguyen N; Peh HY; Shanmugasundaram M; Chandna R; Tee JH; Ong CB; Hossain MZ; Venugopal S; Zhang T; Xu S; Qiu T; Kong WT; Chakarov S; Srivastava S; Liao W; Teh M; Ginhoux F; Wong WSF; Ge R*, “ISM1 protects lung homeostasis via cell-surface GRP78-mediated alveolar macrophage apoptosis”, PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES 119 (4) e2019161119 Published: 2022.


