Single-molecule experiments reveal force capability of artificial molecular motors
April 13, 2022NUS physicists have shown that a single man-made molecular motor can exhibit a force similar to naturally occurring ones which power human muscles.
Molecular motors are a class of molecular machines with nanoscale dimensions that are essential agents of movement in living organisms. They harness various energy sources within the body to generate mechanical motion. A key characteristic is the force generated by a single motor during its self-propelled motion. This force generation capability allows the molecular motor to deliver mechanical work and is a measure of its energy conversion efficiency, which influences its use in potential applications.
The measurement of the force generated by naturally occurring molecular motors, which are usually made of proteins, was achieved two decades ago. However, similar measurements for artificial man-made molecular motors made of DNA (deoxyribonucleic acid) remain a challenge. A research collaboration between the Molecular motors laboratory by Associate Professor Zhisong WANG and the Single-molecule biophysics laboratory by Professor Jie YAN, both from the Department of Physics, National University of Singapore has managed to detect the force generated by a moving DNA molecular motor.
It is difficult to detect the forces created by a single molecular motor in motion for artificial motors because they are small and mostly operate on soft tracks (e.g. double-stranded DNA). Soft tracks are not fixed in position and tend to coil into a circular shape. This affects the motion of the artificial motor. The research team overcame this difficulty by designing and executing in parallel single-molecule experiments that kept the tracks in place at the nanoscale level while also simultaneously detecting the miniscule force created by the moving molecular motor. Using the magnetic tweezers technique, they first assembled an artificial molecular motor and its track under a paramagnetic bead (tool for isolation of biomolecules). They then switched the paramagnetic bead to a force detection mode (see Figure).
The research team successfully applied their method to an autonomous DNA molecular motor (previously developed by Prof Wang’s lab). This bipedal molecular motor is able to “walk” consecutively on its own with a stride length of about 16 nm between each step, providing a maximum force output of around 2 to 3 pN. This measured force output is at a level which is near to naturally occurring molecular motors powering human muscles, indicating a reasonably efficient conversion of chemical energy to mechanical motion.
Prof Wang said, “This study paves the way for the development of applications associated with translational artificial molecular motors which require the generation of forces. Examples include molecular robots and biomimicking artificial muscles. Separately, the single-molecule method established in this work is applicable to force measurement of many other artificial molecular motors with soft tracks.”
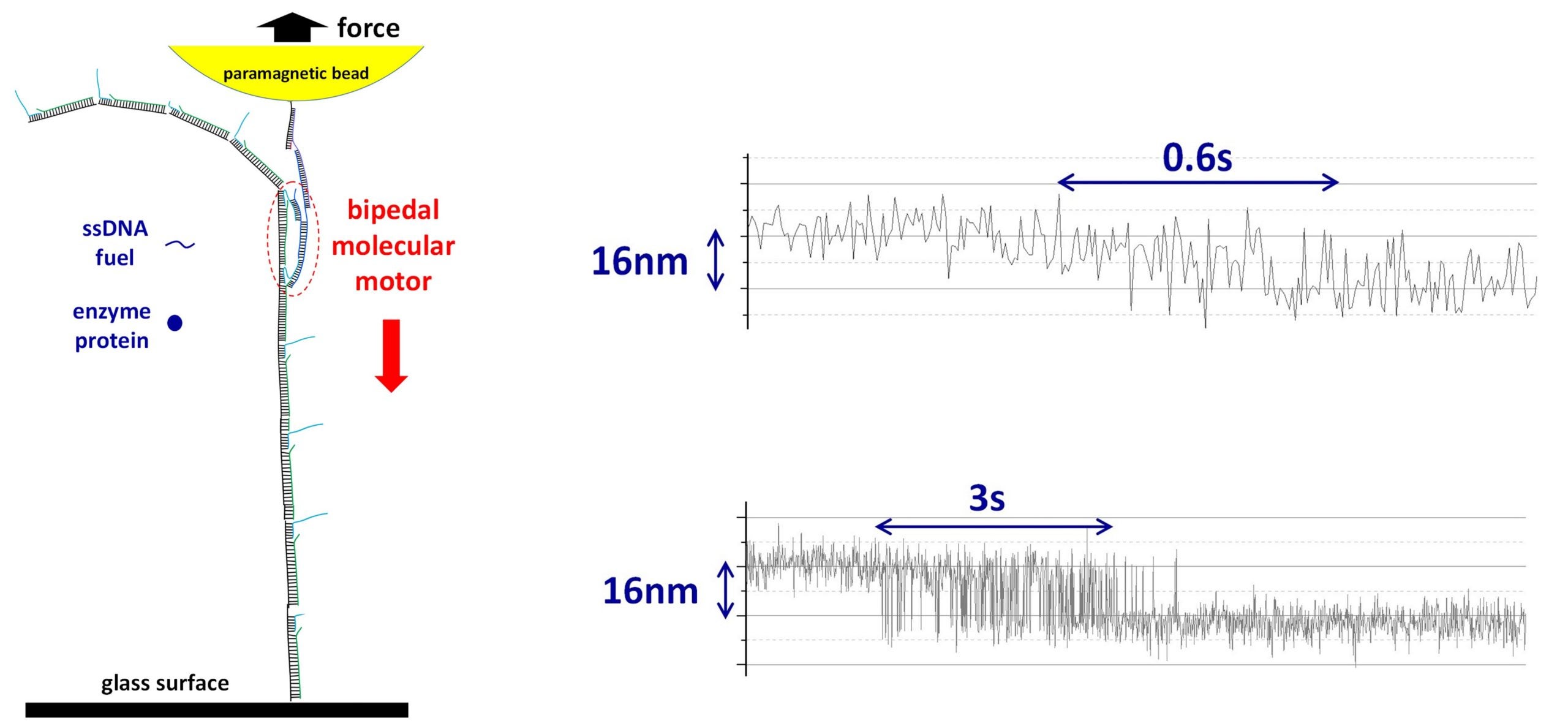
Figure shows the magnetic tweezers setup for the force detection of a single molecular motor (left) and two recorded trajectories showing the motor’s fast stepwise motion (right, against an opposing force of 1.5 pN). The top and bottom panel on the right shows the recorded individual stepping events of the molecular motor with fluctuations in the movement speed typical of single-molecule experiments. A single bipedal nanomotor with a long track is assembled from many short single-stranded DNA molecules under a paramagnetic bead. The motor moves autonomously by consuming a short single-stranded DNA as fuel with the help of a protein enzyme. The molecular motor’s motion is against a backward force applied to the bead, allowing for the measurement of the motor’s load-resisting motion and force output. [Credit: Nanoscale]
Reference
Hu XP; Zhao XD; Loh IY; Yan J; Wang ZS*, “Single-molecule mechanical study of an autonomous artificial translational molecular motor beyond bridge-burning design.” NANOSCALE Volume 13 Issue: 31 Pages: 13195-13207 DOI: 10.1039/D1NR02296B Published: 2021.


