Water clusters in hydrophobic crystalline porous covalent organic frameworks
January 19, 2022NUS chemists have developed hydrophobic porous nanochannels of covalent organic frameworks which can be used to confine and transport water clusters.
Water is essential for daily life and its behaviour can vary in different host materials. For example, water captured in hydrophilic hydrogels can lead to tight locking of water molecules within the material. Hydrophobic materials on the other hand, impose a preconception of having a weak affinity to water molecules as they repel them. This idea precludes the extensive study of water confinement in hydrophobic materials.
A research team led by Professor JIANG Donglin from the Department of Chemistry, National University of Singapore has realised water confinement in hydrophobic microporous materials. They successfully confined water molecules using their specially developed materials, known as hydrophobic crystalline microporous covalent organic frameworks (COFs), by entwining “pseudo-hydrophilicity” strips within a hydrophobic environment. The team further determined the pore size limit for the different topologies (trigonal, tetragonal, and hexagonal channels) in realising water cluster-based adsorption. These findings are important for the future design of water heat-pumps, water transport and separation, as well as realising single-file flow through materials for rapid water transport.
The researchers developed three different material designs, trigonal, tetragonal, and hexagonal microporous covalent organic frameworks with high porosity (see Figure 1). The framework itself is hydrophobic and connected by polar carbon-nitrogen (C=N) imine linkages. These polar sites align vertically to form “pseudo-hydrophilicity” strips within the hydrophobic walls forming channels. Water molecules preferentially interact with the “pseudo-hydrophilic” strips for nucleation. These water molecules then grow into small clusters that fit and then are adsorbed into the channels. As a result, these microporous COFs are able to contain water molecules easily. More than 90% of the free space within a channel can be occupied by water molecules. Interestingly, in these channels, the water does not behave like bulk water. Instead, the extensive hydrogen bonding network present in bulk water is broken down and reconstructed into small clusters when confined in these channels. This could alter water’s intrinsic properties.
The researchers also found that among the three different designs, the behaviour of these water clusters is more sensitive to the trigonal pore design. The adsorption of water vapour can be easily switched off by the addition of a methyl group (CH3) to the trigonal pore walls (see Figure 2). Applying a surface engineering technique to the pores, the amount of water molecules adsorbed can be halved from 93% to 49%. These significant alterations in water adsorption behaviour show that molecular design in COFs is important for controlling water access in the material. Such fine control is rare and difficult to realise in other materials.
With the knowledge gained from this work, the team is planning to further explore the interactions between COFs and water molecules.
Professor Jiang Donglin said, “Our research findings show that small hydrophobic pores can become a central player in the development of game-changing technologies for water uptake, transport and separation and heat-pump energy conversion.”
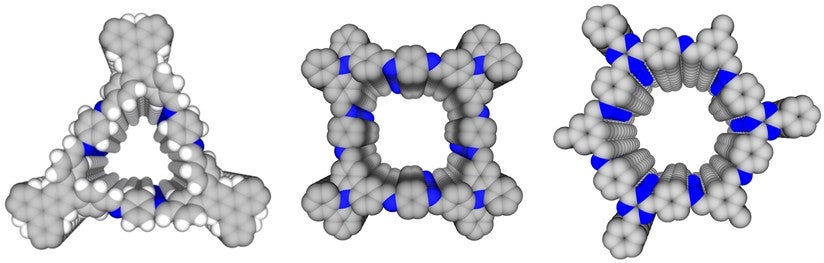
Figure 1: Reconstructed three-dimensional structures of trigonal (HFPTP-PDA-COF, left), tetragonal (TFBCz-PDA-COF, middle), and hexagonal (TTA-TFB-COF, right) covalent organic frameworks. [Credit: Nature Communications]
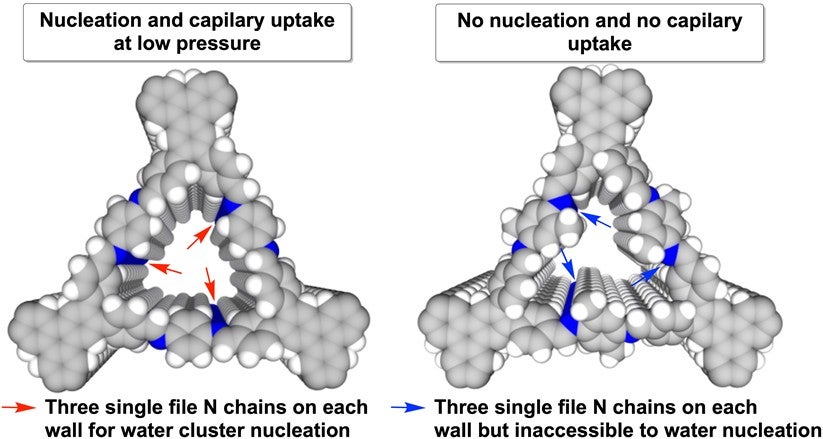
Figure 2: “Hydrophilic” single file C=N chains (blue chains) on walls of vacant HFPTP-PDA-COF (left) and methylated HFPTP-DMePDA-COF (right). The addition of methyl group to the structure (right) strengthens the hydrophobicity of the entire framework, preventing the uptake of water molecules. [Credit: Nature Communications]
Reference
Tan KT; Tao SS; Huang N; Jiang DL*, “Water cluster in hydrophobic crystalline porous covalent organic framework” NATURE COMMUICATIONS Volume: 12 Issue: 1 Article Number: 6747 DOI: 10.1038/s41467-021-27128-4 Published: 2021.


