Governing chromosome segregation
December 13, 2021NUS researchers discovered that a microtubule-associated protein, HMMR, acts as a regulator in controlling chromosome segregation during cell division, an event that is critical for genome stability.
Mitosis is a common but important process in eukaryotic cells, during which genetic information is duplicated and equally separated to daughter cells to ensure the proliferation of the genome while ensuring it keeps its integrity at the same time. This process is regulated by microtubules, which are polymers that form part of the cytoskeleton, and provide structure and shape to eukaryotic cells. Microtubules are classified into three main subgroups, known as kinetochore-microtubules (kMTs), interpolar microtubules (iMTs) and astral microtubules. Decades of studies have dissected the functions and mechanisms of kMTs in regulating chromosome congression (the process of aligning chromosomes on the spindle) and segregation, but the roles of iMTs and its regulatory mechanism are less understood.
A research team comprising Prof LIOU Yih-Cherng and his former Ph.D. student, Dr JIANG Zemin from the Department of Biological Sciences, National University of Singapore found that the microtubule-associated protein HMMR is critical for proper chromosome segregation by regulating the iMTs-formed fibres (see Figure). When the HMMR protein is depleted, the duplicated chromosomes cannot be equally separated. A a result, the separation is delayed, which causes genome instability in daughter cells. This may induce tumorigenesis, a process in which normal cells acquire malignant properties.
By using a laser-based cell ablation system and photoactivation approach, the researchers found that the duplicated chromosomes can be distributed to two daughter cells via the force from microtubules sliding within the iMT-formed fibres, even when it is not connected to the spindle pole on one side. However, loss of HMMR drastically reduced this sliding force, and a much slower velocity of sliding apart has been observed. Further investigation shows that this is because the depletion of HMMR greatly impairs the association with a motor protein called HSET and its movement on the spindle pole. This association and movement are critical to HMMR’s role in transmitting the force from the bridging iMT fibres to the k-fibres, to drag the chromosomes to the daughter cells.
Prof Liou said, “This study identified a novel mechanism of how the bridging fibres formed by iMTs is modulated by microtubule-associated proteins during cell division and may provide evidence linking genome instability with human diseases such as cancer.”
| Visual showing the function of HMMR. The “Go” (in the presence of HMMR, shown in blue) and “Stop” (in the absence of HMMR) represent two situations associated with the microtubule tracks (the bridging fibre) for proper microtubule sliding, whose stability is determined by the association of HSET (indicated by the white and black motor protein) on the mitotic spindle. | 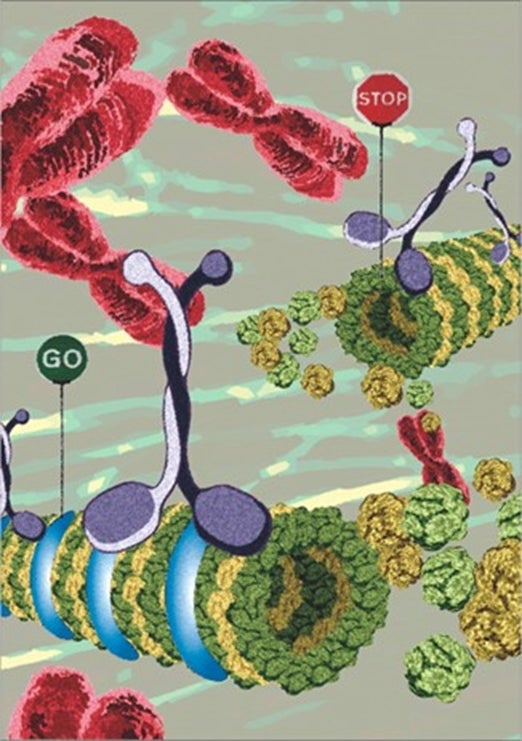 |
Reference
Jiang ZM; Zhang SY; Lee YM; Teng X; Yang QY; Toyama Y; Liou YC*, “Hyaluronan-Mediated Motility Receptor Governs Chromosome Segregation by Regulating Microtubules Sliding Within the Bridging Fiber” ADVANCED BIOLOGY Volume: 5 Issue: 6 Article Number: 2000493 DOI: 10.1002/adbi.202000493 Published: 2021.


