Simple and compact platform for on-demand synthesis of pharmaceutical compounds
WU Jie (Group Leader, Chemistry) June 01, 2021NUS chemists have developed an automated flow synthesis platform by combining continuous-flow synthesis with solid-phase synthesis techniques to enable auto-assembly of a pharmaceutical compound, prexasertib and 23 of its analogues.
Recent advances in end-to-end continuous-flow synthesis are rapidly expanding the capabilities of automated syntheses of small-molecule pharmacophores in flow reactors. There are well-defined production methods for molecules such as peptide and oligonucleotide which have repeating functional units. However, the synthesis of small-molecule based active pharmaceutical ingredients (APIs) remains predominantly a manual process due to its structural diversity. It is challenging to realise a multistep continuous-flow synthesis of APIs due to solvent and reagent incompatibilities, and other associated issues.
A research team led by Prof WU Jie, from the Department of Chemistry, National University of Singapore has developed an automated flow synthesis platform for the production of specific pharmaceutical compounds in a continuous and automated manner by combining two chemical synthesis techniques together. The first technique is continuous-flow synthesis in which chemical reactions are carried out in a continuously flowing manner rather than in batch production. The second technique is the solid-phase synthesis in which molecules are chemically bonded onto an insoluble support material and grown in a stepwise manner. In the new technique, known as solid phase synthesis-flow (SPS-flow), the target molecule is developed on a solid supporting material as the reaction reagent flows through a packed-bed reactor. The entire reaction process is automatically controlled by a computer-based chemical recipe file programmed using the LabVIEW software.
With the SPS-flow platform, prexasertib with a yield of up to 65 percent was obtained within 32 hours of continuous automated execution. Conventional methods would require a six step process that would take much longer and requires cumbersome human manipulation to obtain the pharmaceutical product.
The research team also directly used or slightly modified the chemical recipe file to obtain 23 derivatives of prexasertib. These derivatives are molecules having high chemical similarity but differ from the original molecule in a certain aspect. The capability to easily obtain these derivatives is important during the drug discovery and design process where structure–activity relationship studies based on initial core structures play an important role for the selection of clinical candidates. Conventional methods which mainly relied on late-stage diversification mean that only specific sites of the molecule can normally be functionalised. The new technique does not have this limitation.
Prof Wu said, “Analysis of the top-selling 200 small-molecule pharmaceuticals indicates that automated SPS-flow synthesis could potentially be applied to a wide range of pharmaceutical molecules. However, there is still a long process before the SPS-flow synthesis can be applied in drug manufacturing and drug development. Future studies will target the development of a fully automated and portable system for API production at a larger scale which is more suitable for a manufacturing environment. It will also apply the SPS-flow technology in lead optimisation to speed up the drug discovery process.”
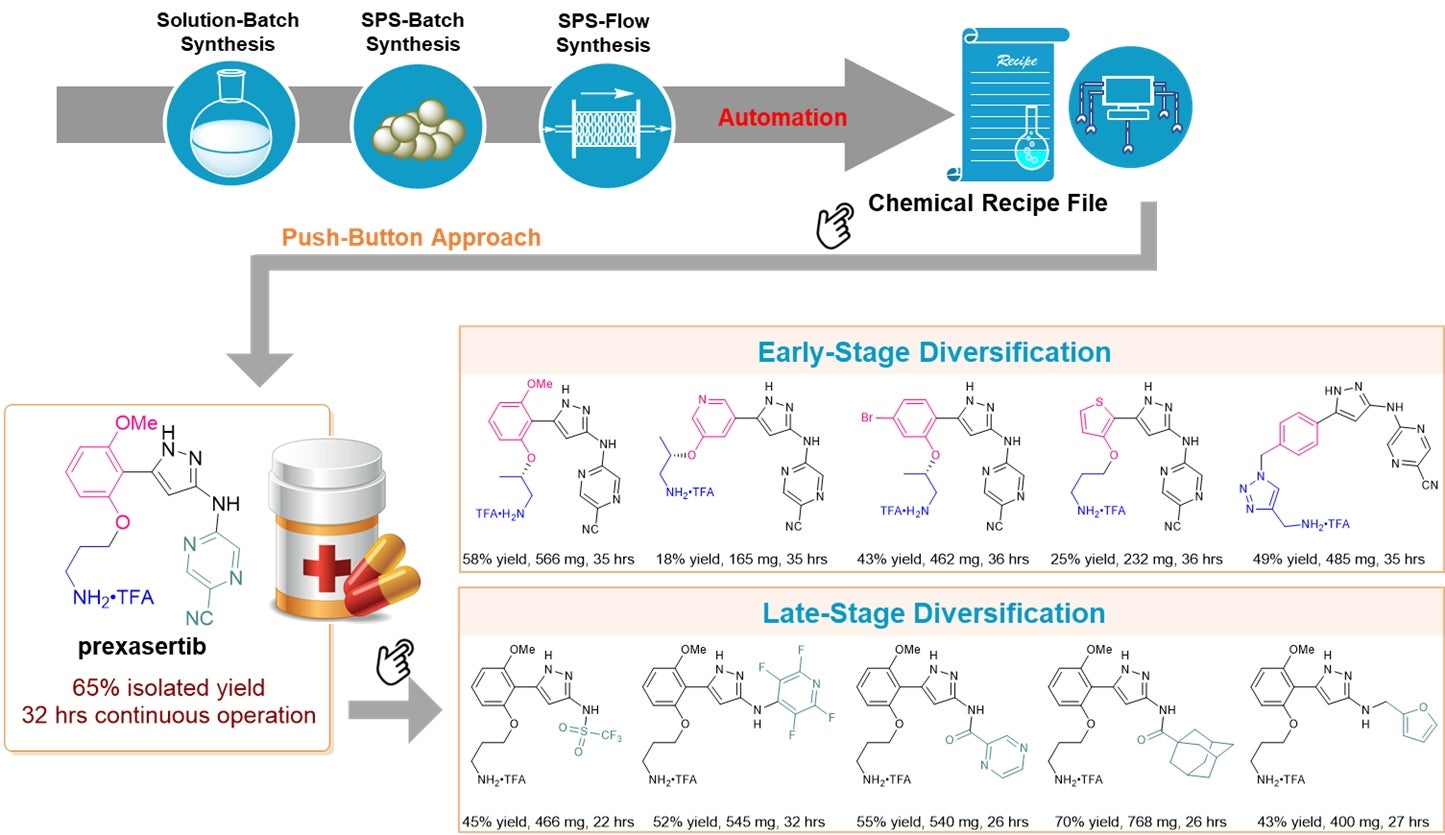
Figure provides an overview of the workflow for the SPS-flow enabled automated synthesis of prexasertib and its derivatives. Through the computer-based chemical recipe file, the processing of the pharmaceutical molecule can be automatically controlled. It also allows for early-stage and/or late-stage diversification of the lead compound easily.
Reference
Liu CG; Xie JX; Wu WB; Wang M; Chen WH; Idres SB; Rong JW; Deng LW; Khan SA*; Wu J*, “Automated synthesis of prexasertib and derivatives enabled by continuous-flow solid-phase synthesis” NATURE CHEMISTRY Volume 13 DOI: 10.1038/s41557-021-00662-w Published: 2021.
Comments by Dr Kevin P. Cole from Eli Lilly on the research paper.


