Synthesis of a sidewall fragment of a (12,0) carbon nanotube
CHI Chunyan (Group Leader, Chemistry) April 22, 2021NUS chemists have developed a strategy for the atomically precise synthesis of fully conjugated zigzag-edged carbon nanobelts (CNBs). The obtained molecule, known as octabenzo[12]cyclacene, is acknowledged as one of the first fully characterised synthetic segments of zigzag-edged (12,0) carbon nanotube. Such molecular structures have been elusive targets for synthetic chemists for the past 35 years.
Single-walled carbon nanotubes (SWCNTs) are a special class of carbon materials comprising sheets of graphene in a hollow tube structure with walls of one atom thickness. They are regarded as one of the most promising materials for the development of next generation nanoelectronic devices. However, current production methods such as arc-discharge and laser vaporisation, are unable to achieve atomically precise synthesis of SWCNTs which affects their electrical and optical properties. As an alternative method, research has focused on CNBs which are belt-shaped molecules consisting solely of benzene rings fused together in a circular manner. These CNB molecules could potentially serve as a seed for the growth of structurally well-defined carbon nanotubes. In recent years, there has been a revival of interest in bottom-up organic synthesis of CNBs. CNBs with different configurations such as arm-chaired and chiral edges have been synthesised and fully characterised, respectively. However, the synthesis of a unique configuration involving the fragments of zigzag edged (n,0) CNTs remains elusive (see Figure (a)).
A research team led by Prof CHI Chunyan from the Department of Chemistry, National University of Singapore, has developed a strategy which combines both thermodynamic stabilisation and kinetic protection to achieve atomically precise synthesis of a zigzag-edged (12, 0) SWCNT segment. The synthesis was accomplished by using Diels–Alder addition two times to first construct a strain-free precursor, followed by reductive deoxygenation to obtain a fully conjugated, strained CNB. A concept known as benzo-annulation was applied to increase the resonance stabilisation energy so that thermodynamic stability of the final compound can be achieved. Meanwhile, attachment of substituents onto the zigzag edges would kinetically prevent cycloaddition reactions which may originally destroy the conjugated backbone structure.
The research team used several advanced characterisation tools to investigate the structure of the octabenzo[12]cyclacene molecule which they had obtained. Using single crystal x-ray diffraction, they found that the molecule adopts a highly symmetric cylinder-like geometry (Figures (c) and (d)), similar to that of a carbon nanotube. A crystal structure visualisation, exploration and analysis software (Mercury) was used to measure the inner diameter of this nanobelt which is about 9.2 Å. The researchers also performed computational strain analysis on the molecular structure and their results suggest that the phenyl groups at the zigzag edges play an important role in providing stability by preventing further hydrogenation reactions during the formation of the nanobelt structure.
Prof Chi said, “Our synthetic approach and stabilising strategy developed in this work can pave the way for the building of new types of carbon nanostructures and carbon nanotubes for various applications in electronics and photonics.”
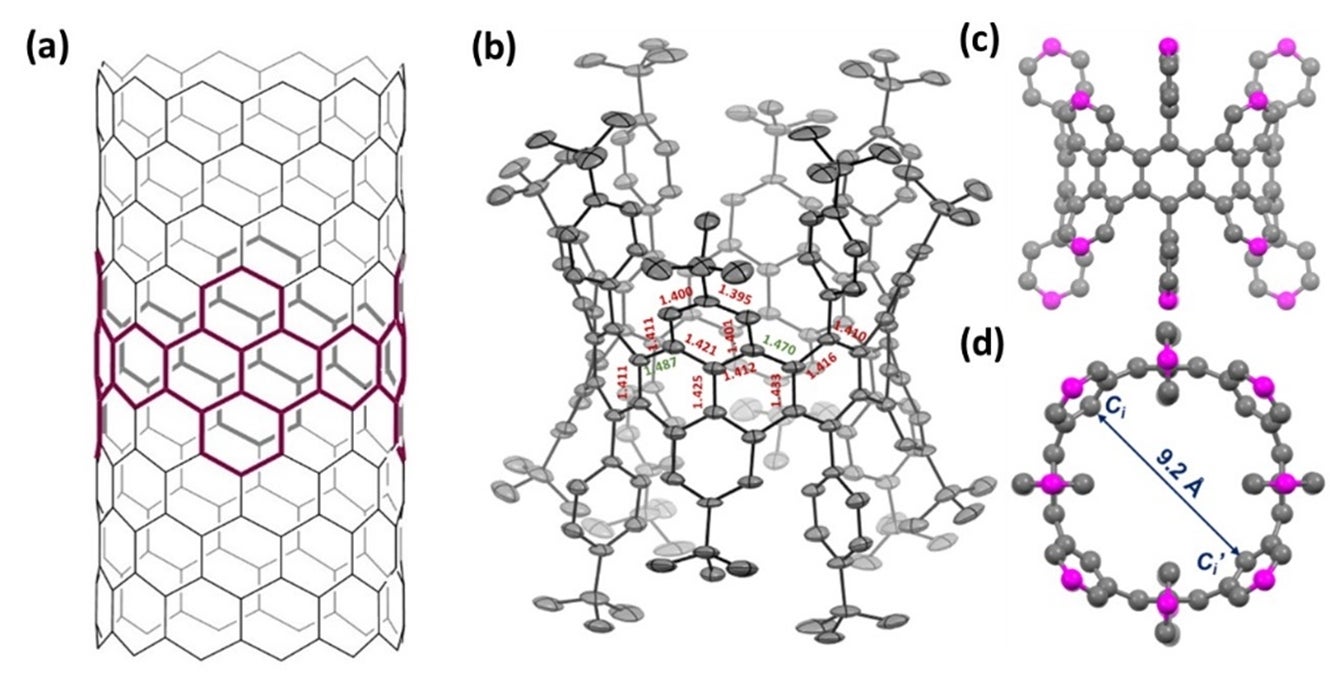
Figure: (a) Highlighted parts of the visual shows the sidewall fragment of a (12,0) carbon nanotube (octabenzo[12]cyclacene) which was synthesised as part of this work. X-ray crystallographic structures of the compound showing the (b) bond length analysis, (c) side view and (d) top view. The atoms highlighted in pink were attached with t-butyl groups which were omitted for clarity. [Credit: HAN Yi]
Reference
Han Y; Dong S; Shao J; Fan W; Chi C*, “Synthesis of a Sidewall Fragment of a (12,0) Carbon Nanotube”, Angewandte Chemie-International Edition Volume: 60 Issue: 5 Pages: 2658-2662 DOI: 10.1002/anie.202012651 Published:2021.


