Divergent synthesis of bicyclic medium-sized ring structures
ZHAO Yu (Group Leader, Chemistry) October 26, 2020NUS chemists have discovered catalyst-controlled divergent reactions to synthesise three different classes of medium-sized bicyclic compounds from the same starting materials for the development of therapeutic drug molecules.
Medium-sized ring-containing natural products have been exploited by nature to address various challenging biological targets. However, synthetic compounds of this type are rarely utilised in drug discovery, largely due to the lack of reliable synthesis methods. Intermolecular higher-order cycloaddition, in which two starting materials are “stitched” together at both ends, provides great potential to build complex cyclic compounds from simple building blocks. Unfortunately, such transformations to prepare medium-sized rings are often plagued with competitive reaction pathways and low levels of site- and stereo-selectivity. Methods to realise precise control for the synthesis of this class of compounds represent an unsolved challenge in organic chemistry.
A research team led by Prof ZHAO Yu, from the Department of Chemistry, NUS has taken this challenge in the past few years and achieved a few highly efficient preparations of different 9-10-membered heterocycles. Key to their success is the continuous effort to identify novel and suitable cross partners that can undergo efficient cycloaddition to selectively prepare medium-sized rings (in preference to the smaller ring analogues). Recently, they came up with an intriguing catalytic system to prepare new bicyclic compounds (shown in figure below). The most significant aspect of this chemistry was that not one, but three different classes of bicyclic medium-sized ring compounds were obtained with high efficiency and stereo-selectivity from the same set of starting molecules. The choice of different ligands for the palladium catalytic system was the determining factor for such switch of reactivities. Mechanistic studies and density functional theory calculations also provided a comprehensive picture on how these reactions work and where the selectivity stems from.
Prof Zhao said, “The initial research plan was to develop a catalyst which would allow for the selective formation of one class of bicyclic medium-sized ring compounds that was never accessed before, so it would represent a new chemical space for drug discovery. However, in the course of our work, we found that by switching the reaction conditions, three skeletally different medium-sized bicyclic compounds with high conversion ratios could be obtained. Such divergent syntheses with high efficiency will greatly enhance chemists’ ability to access new chemical space for delivering a larger library of potential drug candidates.”
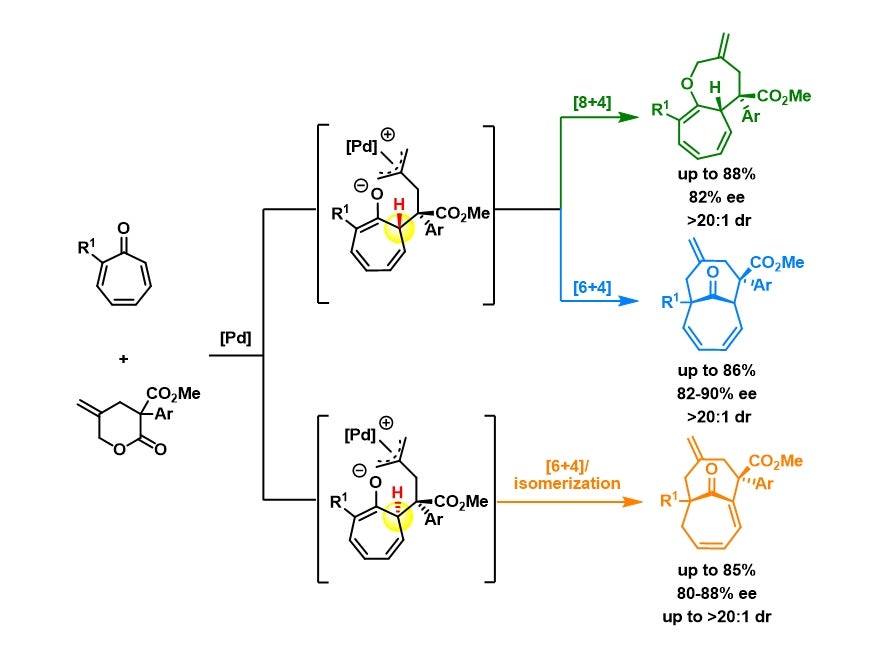
Figure shows the reaction pathways of the divergent synthesis of three classes of compounds. Using the same set of starting materials and palladium catalyst but subjected to different reaction conditions, it results in three structurally different medium-sized bicyclic compounds, shown in green, blue and orange.
Reference
Yang LC; Wang YN; Liu R; Luo Y; Ng XQ; Yang B; Rong ZQ; Lan Y; Shao Z; Zhao Y*, “Stereoselective access to [5.5.0] and [4.4.1] bicyclic compounds through Pd-catalysed divergent higher-order cycloadditions” NATURE CHEMISTRY Volume 12 Pages: 860-868 DOI: 10.1038/s41557-020-0503-7 Published: 2020.


