Electrochemical reduction of carbon dioxide to ethanol
Jason YEO (Group Leader, Chemistry) May 04, 2020NUS scientists have discovered a new mechanism for selective electrochemical reduction of carbon dioxide (CO2) to ethanol using copper-silver (Cu-Ag) composite catalysts.
Electrochemical reduction of CO2 to fuels and chemicals, when powered by renewable electricity, is a step forward in alleviating carbon emissions. Copper (Cu) materials are choice catalysts for this process because they have the highest electrochemical activities towards multi-carbon products. However, their selectivity towards ethanol (C2H5OH), a valuable fuel and chemical feedstock, is always lower than towards ethylene (C2H4). The preference towards production of ethylene compared to ethanol arises from the CO dimerisation mechanism for producing C2 molecules from CO2, where the formation of ethylene, which has a lower energy barrier, is favoured over ethanol.
A research team led by Prof YEO Boon Siang, Jason from the Department of Chemistry at NUS, in collaboration with a team led by Dr Federico CALLE-VALLEJO from the University of Barcelona, has shown that an influx of CO molecules, provided by silver (Ag) co-catalysts, activates an otherwise-locked mechanistic pathway on Cu which converts CO2 gas to ethanol.
A series of Cu-Ag composite catalysts, fabricated from a mixture of oxide-derived Cu nanowires and Ag powders, were tested for their electrochemical CO2 reduction activities. During CO2 reduction, Ag converts CO2 to CO and these CO molecules migrate to the Cu active sites for further reduction to hydrocarbons (ethylene) and alcohols (ethanol). The researchers varied the Ag/Cu ratio and the Ag particle sizes in the composites to increase the CO influx from Ag to the active sites on the Cu material. The experimental results showed that the increased CO influx enhanced the production of ethanol by up to five times, with little impact on ethylene production. Theoretical simulations on the reaction mechanism show that instead of the CO+CO step which results in ethylene formation, the CO+CHx step was the dominant C-C bond formation step at the Cu-Ag interface. Ethanol was the sole product when the reaction proceeds through the CO+CHx step, which was found to occur on active sites different from those that facilitated ethylene formation via the CO+CO step.
Further plans arising from this finding by the research team include maximising the active sites through catalyst design and upscaled production using a high throughput flow cell configuration.
Prof Yeo said, “The concept that a previously closed pathway can be opened by an influx of intermediates, as demonstrated in this work, opens new possibilities to uncover new synthetic mechanisms which might previously be inaccessible.”
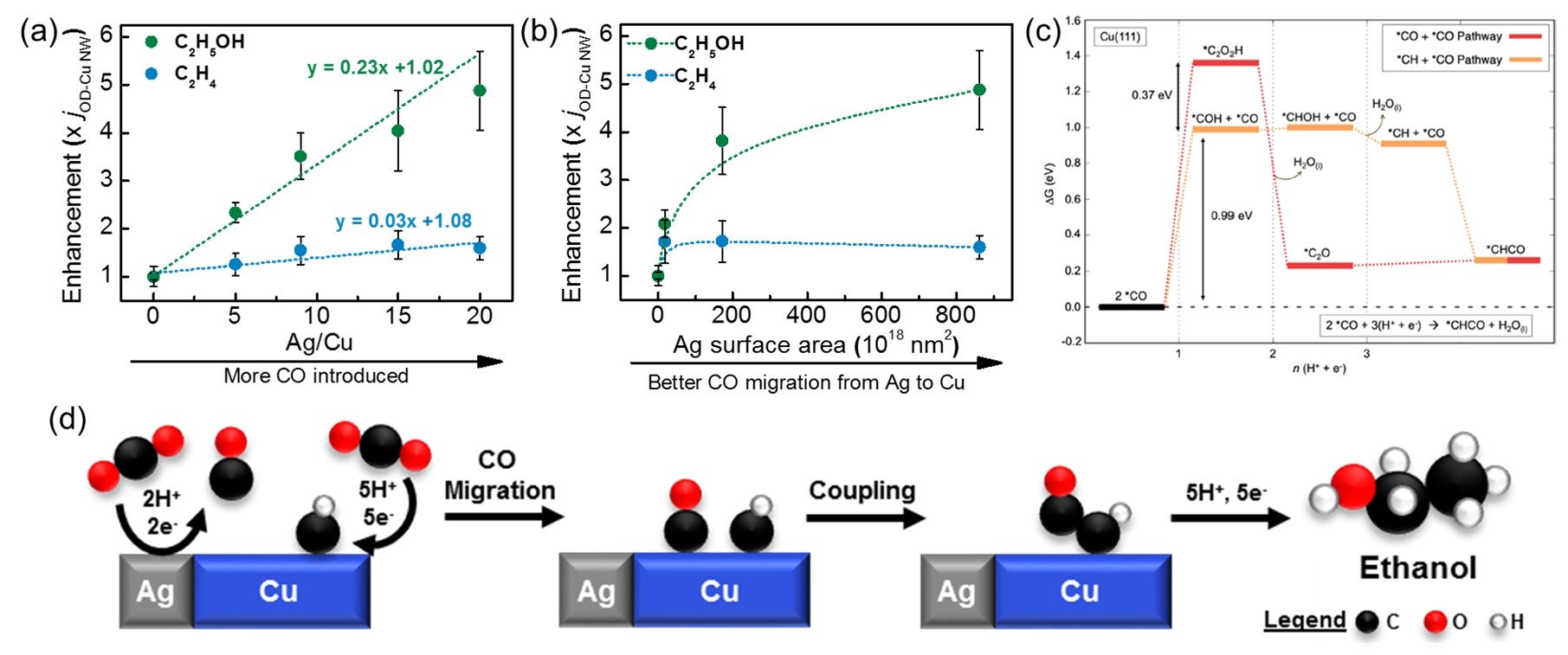
Graphs showing how the enhancement of ethanol (green) and ethylene (blue) production on copper-silver (Cu-Ag) composites varies with different (a) Ag/Cu ratios and (b) Ag particle sizes. A five-fold enhancement was observed for ethanol, while ethylene was not significantly impacted. (c) Energy level diagram showing CO+CO (red) and CO+CH (orange) coupling steps on Cu (111). A lower barrier is required for CO+CH coupling, making it more favourable than the CO+CO step. (d) Scheme showing the mechanism for CO2 reduction to ethanol on Cu-Ag composites via CO+CH coupling. [Credit: ACS Catalysis]
Reference
Ting LRL; Pique O; Lim SY; Tanhaei M; Calle-Vallejo F*; Yeo BS*. “Enhancing CO2 Electroreduction to Ethanol on Copper–Silver Composites by Opening an Alternative Catalytic Pathway” ACS CATALYSIS Volume: 10 Issue: 7 Pages: 4059-4069 DOI: 10.1021/acscatal.9b05319 Published: 2020.


