Imaging structural changes in catalysts during reaction conditions
Utkur MIRSAIDOV (Group Leader, Biological Sciences and Physics) May 15, 2020Scientists from National University of Singapore (NUS) imaged the structural changes in noble metal catalysts during carbon monoxide (CO) oxidation, showing that the catalysts switch between inactive and active states depending on temperature.
Significant research efforts have been devoted towards the development of catalysts with higher performance as they can reduce the energetic cost of maintaining a chemical reaction. However, such efforts are often limited by a lack of detailed insights into the structural changes of the catalysts during these chemical reactions. Catalysts can change their structure according to the reaction conditions, but most of the available analytical tools are not able to capture these changes under realistic operating environments.
A research team led by Prof Utkur MIRSAIDOV from the Department of Physics and the Department of Biological Sciences, NUS have demonstrated the direct imaging of structural changes in Palladium (Pd) nanoparticles which act as catalysts during the CO oxidation reaction at atmospheric pressures using state-of-the-art operando transmission electron microscopy (TEM). The observations showed that depending on the temperature, the catalysts have two distinct structures. The transformation from a structure dominated by low index facets to a rounded structure due to increasing temperature is associated with an inactive to active catalyst transition. Reducing the temperature reverses the structural change and the nanoparticles revert back to their inactive structure. Thermodynamic modelling results from Dr Alexander GENEST and his team at the Institute of High Performance Computing, Agency for Science, Technology and Research confirmed their experimental findings that the low temperature structure contains less active sites on its surface and is therefore less active when compared to the high temperature structure.
Dr See Wee CHEE, the first author of the journal paper, said, “These observations of reversible transformations in catalysts have important implications for the catalysis community. The conventional approach for catalyst characterisation involves removing catalysts from their working conditions for subsequent investigations. Our results show that the active structure of the catalyst may not be retained during such a process and emphasises the important role operando studies play in the design and development of new catalysts.”
The group is planning to extend these studies towards more complex nanostructures and reactions that are relevant to the chemical industry.
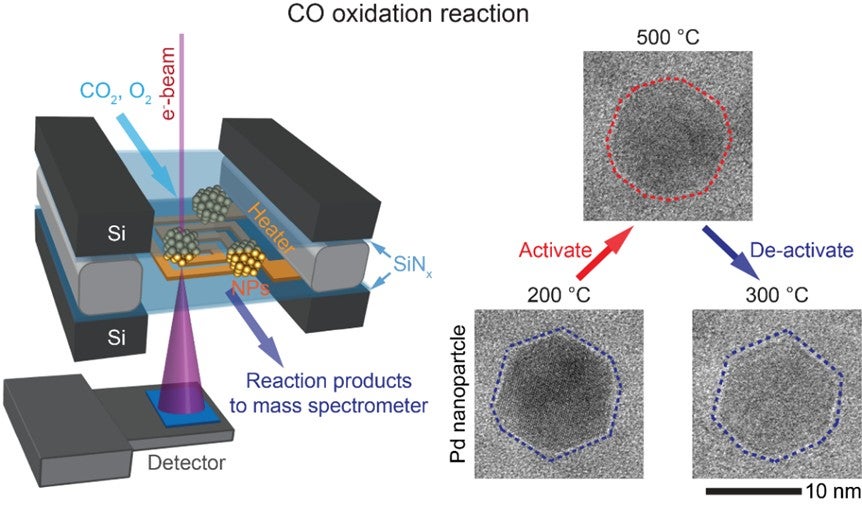
Figure (left) shows the setup used in the capturing of reversible transformations in Palladium (Pd) nanoparticles during carbon monoxide (CO) oxidation reaction with operando transmission electron microscopy (TEM). These transformations determine the catalytic activity of the nanoparticles. (Right) TEM images of Pd nanoparticles under different temperature conditions.
Reference
Chee SW; Arce-Ramos J; Li W; Genest A; Mirsaidov U*, “Structural Changes in Noble Metal Nanoparticles during CO Oxidation and Their Impact on Catalyst Activity” NATURE COMMUNICATIONS Volume: 11 Page: 2133 DOI: 10.1038/s41467-020-16027-9 Published: 2020.


