(Group Leader, Biological Sciences)
NUS biophysicists have discovered new conformational changes and structural dynamics of the dengue virus during their transmission to human hosts.Dengue infection is a mosquito-borne tropical disease caused by the dengue virus. It affects about 400 million people worldwide and is one of the most common mosquito-borne viral diseases in Singapore. Dengue infection can be fatal and there is no effective treatment available. It is transmitted through the bite of the female mosquito and cannot be spread directly from person to person through contact. A mosquito is infected when it feeds on a dengue-infected person and the virus is transmitted to other people when the infected mosquito bites them.Prof Thorsten WOHLAND from the Departments of Biological Sciences and Chemistry, NUS and his research team have discovered that the structural dynamics, but not the specific virus morphologies, are correlated to the ability of certain strains of dengue viruses to establish an infection (infectivity). While the structures of the dengue virus change dramatically in the mosquito and human hosts due to temperature differences, neither infectivity nor the structural dynamics of the virus envelope change. The infectivity and structural dynamics of the dengue virus are only affected when it is exposed to fever-like temperatures of 40°C. At 40°C, there is a decrease in virus infectivity by more than three times and a 2.5 times reduction in virus structural dynamics. These findings hold enormous potential for devising new strategies to block dengue infection by targeting the virus structural dynamics.When the dengue virus enters a human host, there is a change in temperature from about 25°C (inside the mosquito) to 37°C (inside a healthy human) or 40°C (inside a dengue-infected human). This temperature difference causes the dengue virus to “puff” up and to become approximately three times less infective. However, this loss of virus infectivity happens only after the dengue virus experienced a temperature of 40°C. The team also found that in the absence of divalent cations (Ca2+, Mg2+), the dengue virus will remain in its “puffed” state even when the temperature reverts to 25°C. This happens when the virus is transmitted back to a mosquito from a human host. The dengue virus will only contract partially in the presence of divalent cations. By monitoring the influence on the dengue virus due to temperature and the presence of divalent cations, this work has helped to establish the correlation between virus structural dynamics and virus infectivity.
Prof Wohland said, “Our research group is expanding plans for in-depth investigation of dengue and its life cycle. We are also extending these strategies to other closely related viruses to provide new clues for the development of antiviral strategies.”
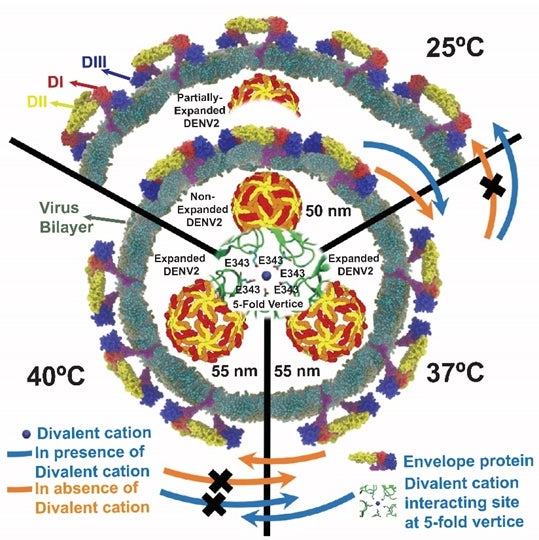
Figure showing the morphological and structural changes in the dengue virus at different temperatures. At 25°C, envelope proteins at the membrane of the dengue virus remain in close proximity and the diameter of the virus is 50nm. Upon increase in temperatures to greater than or equal to 37°C, the distance between envelope proteins and virus increases. This leads to the “puffing” of the dengue virus and its diameter increases to 55nm. The team discovered that when there is a decrease in the temperature (back to 25°C), the dengue virus will partially contract only when there are divalent cations present in its surroundings. DI, DII and DIII are three exposed domains of the envelope protein on the virus surface.
References
Sharma KK; Lim XX; Tantrimudalige SN; Gupta A; Marzinek JN; Holdbrook D; Lim XYE; Bond JP; Anand GS; Wohland T*, “Infectivity of dengue virus serotypes 1 and 2 is correlated to E protein intrinsic dynamics but not to envelope conformations” STRUCTURE PII: S0969-2126(18)30462-3 DOI: 10.1016/j.str.2018.12.006 Published: 2019.
Sharma KK; Marzinek JK; Tantirimudalige SN; Bond PJ; Wohland T*, “Single-molecule studies of flavivirus envelope dynamics: Experiment and computation” PROGRESS IN BIOPHYSICS & MOLECULAR BIOLOGY PII: S0079-6107(18)30181-0 DOI: 10.1016/j.pbiomolbio.2018.09.001. Review.
Lim XX; Chandramohan A; Lim XYE; Bag N; Sharma KK; Wirawan M; Wohland T; Lok SM; Anand GS*, “Conformational changes in intact dengue virus reveal serotype-specific expansion.”, NATURE COMMUNICATIONS Volume: 8, Article number: 14339 DOI: 10.1038/ncomms14339 Published: 2017.



