Oxygen migration at the heterostructure interface
ARIANDO (Group Leader, Physics) January 10, 2019NUS physicists have developed a methodology to control the electromigration of oxygen atoms in the buried interfaces of complex oxide materials for constructing high mobility oxide heterostructures.
Oxide heterostructures, which are composed of layers of different oxide materials, exhibit unique physical properties at their interfaces which do not usually exist in their parent compounds. An example is the interface comprising an insulating film of lanthanum aluminate (LaAlO3, abbreviated as LAO) on insulating strontium titanate single crystal (SrTiO3, abbreviated as STO). This interface shows various unique material properties, such as conductivity, magnetism and two-dimensional superconductivity, which are not observed in their bulk forms. Oxygen vacancies in STO are known to play an important role in influencing these properties, particularly for interfaces which can be synthesised at room temperature. However, the underlying mechanisms influencing these emergent properties by the oxygen vacancies at the interface of the two different materials is still unclear.
A research team led by Prof ARIANDO from the Department of Physics, and the Nanoscience and Nanotechnology Initiative (NUSNNI), NUS has developed a new and unique technique based on the electrolyte field effect to control oxygen vacancy concentration at the interface of LAO/STO heterostructures. They discovered that there is an enhancement in the electron mobility of the heterostructure when oxygen vacancies at the oxide interface become occupied (filled). This effect could potentially be used to construct high-performance semiconductor devices.
The researchers used an electrolyte as the dielectric material on the LAO/STO heterostructure and applied a negative voltage to it. This creates a strong electric field that causes the oxygen atoms in the LAO layer to migrate into the oxygen-deficient STO at the interface region. The concentration of oxygen vacancies at the STO interface is reduced and this changes the energy band structure of the heterostructure, enhancing electron mobility. In this experimental setup, the amorphous LAO surface layer acts as a barrier, preventing chemical reactions from occurring between the sample surface and the electrolyte.
Prof Ariando said, “Our finding provides further clues to understand the mechanism of electrolyte field effect, and opens a new avenue for constructing high-mobility oxide interfaces which can be synthesised at room temperature.”
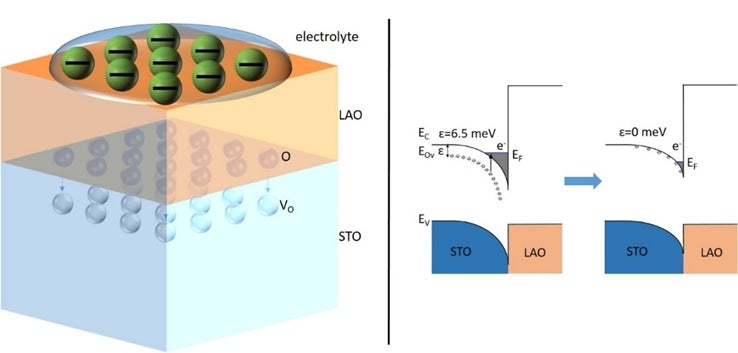
(Left) Schematic illustration of the operation of LAO/STO electrolyte field effect device. The presence of high electric field drives the oxygen atoms (O) migration into the STO layer to fill some of the vacancies (VO). (Right) Schematic band diagrams of the LAO/STO interfaces showing the change in interfacial energy band structures as a result of electrolyte field effect. [Credit: Physical Review Letters]
Reference
Zeng SW*, Yin XM, Herng TS, Han K, Huang Z, Zhang LC, Li CJ, Zhou WX, Wan DY, Yang P, Ding J, Wee ATS, Coey JMD, Venkatesan T, Rusydi A, Ariando*, “Oxygen Electromigration and Energy Band Reconstruction Induced by Electrolyte Field Effect at Oxide Interfaces” PHYSICAL REVIEW LETTERS Volume: 121, Pages:146802 DOI: 10.1103/PhysRevLett.121.146802 Published: 2018.


