Real-time imaging of chemical processes
July 24, 201824 Jul 2018. NUS scientists observe the real-time formation of hollow structures in the galvanic replacement (GR) reaction between silver and gold with nanometre resolution, gaining insights on the mechanisms behind the structural transformations.
Hollow bimetallic nanoparticles have a high surface area to volume ratio and are good candidates for developing catalytic materials because they allow for more interactions between the reactants and the catalyst surface. The GR reaction is a widely-used approach for forming such nanoparticles. During the reaction, structural changes are driven by a difference in electrochemical potential between two different metals in a solution, which leads to the preferential corrosion of one metal over the other. However, it is still a challenge to produce hollow bimetallic nanoparticles with uniform size and shape by chemical synthesis because the precise roles of the growth processes remain unclear.
A team led by Prof Utkur MIRSAIDOV from both the Department of Physics and Department of Biological Sciences, NUS has gained a mechanistic understanding of the structural changes during the formation of hollow silver nanocubes as they react with gold ions in a solution. This was achieved using liquid cell transmission electron microscopy (LC-TEM), which is a new technique in transmission electron microscopy that allows scientists to examine processes in liquids with nanometre resolution. They observed in real-time that the silver nanocubes become hollow via the formation, growth, and coalescence of internal voids. During the replacement reaction, metallic gold is deposited on the silver nanocube surface with concurrent dissolution of silver into solution. The common assumption is that, the silver core gradually hollows out through a hole in the gold shell. However, the team found that during the reaction, voids form at the interface between the silver and gold metals, often near the corners of the nanocubes and then, the hollowing proceeds inward.
These LC-TEM observations imply that another process, the Kirkendall effect (KE), also contributes to nanoparticle hollowing. KE occurs at bimetallic interfaces because the two metal diffuse into each other at different rates. It results in the formation of voids on the side of the faster diffusing metal, which is consistent with the LC-TEM observation. The team further characterised the changes in the structural transformations of the nanoparticles as a function of the quantity of gold ions present in the solution and the temperature of their surroundings, which all point towards a coupling between KE and GR during the hollowing process. The voids grow faster with increasing temperature, indicating faster atomic diffusion and is consistent with the behaviour expected for KE.
Explaining the significance of the findings, Prof Mirsaidov said, “We are very excited by these results. Our team is the first to directly observe in real-time the KE as a transient, intermediate stage in the hollowing reaction between silver and gold. This approach can potentially be extended to the study of other liquid phase reactions at elevated temperatures, which brings us closer to actual reaction conditions.”
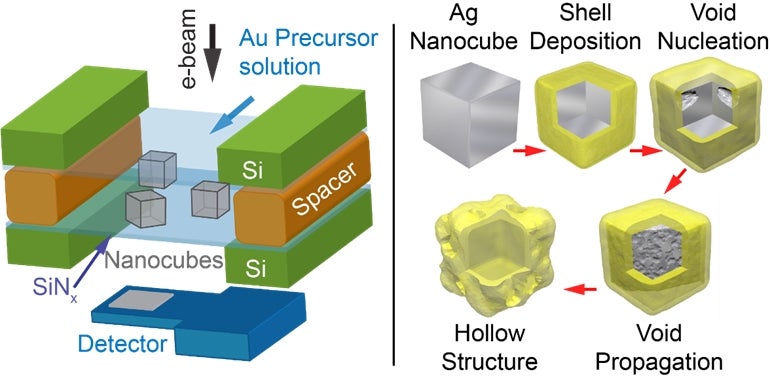
(Left) Figure shows a schematic of the in situ LC-TEM setup involving silver (Ag) nanocubes in a solution of gold (Au) ions. (Right) Mechanism showing the structural transformation during the galvanic replacement reaction.
Reference
Chee SW; Tan SF; Baraissov Z; Bosman M; Mirsaidov U*, “Direct observation of the nanoscale Kirkendall effect during galvanic replacement reactions” NATURE COMMUNICATIONS Volume: 8 Article Number: 1224 DOI: 10.1038/s41467-017-01175-2 Published: 2017.


