Fluorescent probes to study cellular activity
November 07, 20177 Nov 2017. NUS chemists have recently developed selective probes for cytosolic phospholipase A2 (cPLA2) to determine enzyme levels and activity.
cPLA2 is an important enzyme heavily utilised in regulating inflammatory responses in the body. It has been gaining significant interest medically, with increasing links of its involvement in inflammatory and neurological diseases such as Alzheimer’s disease and multiple sclerosis. The ability to image and correctly identify cPLA2 in biological systems is important in understanding the mechanistic pathways involved in these diseases. A team led by Prof LAM Yulin from the Department of Chemistry, NUS has discovered some fluorescent compounds that could image cPLA2. These include an inhibitor (compound which reduces the enzyme activity) and a substrate (compound which the enzyme acts on) that exhibit fluorescence.
By mimicking the structure of a well-known inhibitor of cPLA2, arachidonyl trifluoromethyl ketone (AACOCF3), the research group has successfully attached a fluorescent organic dye (coumarin) onto the carbon chain end of AACOCF3. This created a fluorogenic form of AACOCF3 which retained its native inhibitory activity towards cPLA2. Preliminary studies performed on the newly designed compound showed its ability to differentiate between cells containing different levels of cPLA2. Concurrently, this newly developed probe was able to inhibit cPLA2, providing a dual role of imaging and inhibition. This allows biochemists to directly detect the enzyme at the cellular level while effecting an intended biological response.
Encouraged by these results, the research group expanded their studies by developing another probe to measure cPLA2 activity. The conventional assay of cPLA2 activity uses a radioactive compound as a substrate. However, the use of such assays is highly undesirable due to the hazards surrounding radioactive materials. To circumvent this problem, various calorimetric and fluorogenic assay kits for the measurement of cPLA2 activity are now commercially available. However, these assays can also detect other enzymes in the PLA2 family and are not selective only to cPLA2. It would be useful to have an alternative probe, which is specifically targeted at cPLA2.
By adopting a similar approach, fluorogenic coumarin and fluorescein (another fluorescent organic dye) moieties were attached onto phosphatidylcholine (a substrate of cPLA2). This causes a non-radiative internal transfer of energy between one dye to the other when the light sensitive part is irradiated at its excitation wavelength; a phenomenon known as Förster resonance energy transfer (FRET). This new substrate probe was found to be highly selective to cPLA2, with no loss of native activity and suitable for inhibitor screening assays.
The group is currently investigating the effects of attaching fluorescent chemical compounds of different colours onto both the inhibitor and substrate probes so as to widen their applications.
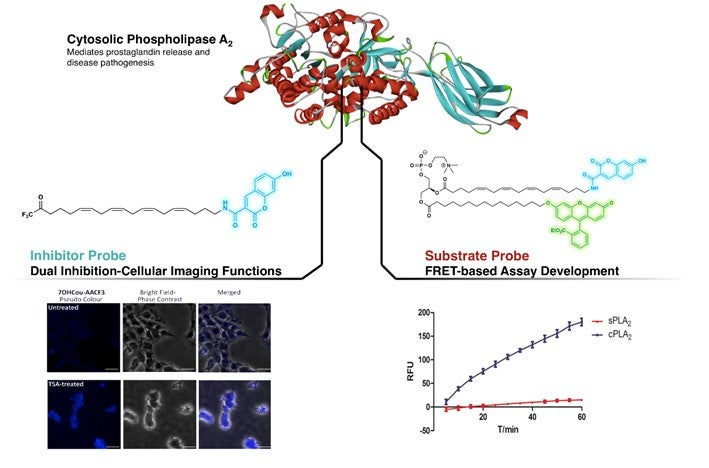
Figure shows the targeting of cPLA2 by the newly designed inhibitor and substrate probe. Left: Identifying differences in cPLA2 level in untreated and Trichostatin A (TSA, an inhibitor compound)-treated SHSY5Y cells. Right: Demonstrating higher selectivity for cPLA2 against sPLA2 (another member of the phospholipase A2 family) by FRET-based assay using the substrate probe. [Image credit: NG Cheng Yang, CAO Xujun]
Reference
Ng CY; Kwok TXW; Tan FCK; Low C-M*; Lam Y*, “Fluorogenic Probes to Monitor Cytosolic Phospholipase A2 Activity” CHEMICAL COMMUNICATIONS Volume: 53, Issue:11, 1813-1816, DOI: 10.1039/C6CC09305A Published: 2017.


