Maintenance of outer membrane lipid asymmetry in bacteria
CHNG Shu Sin ((Group Leader, Chemistry) ) May 24, 201724 May 2017. NUS scientists have discovered the key roles for auxiliary proteins in a transporter that maintains outer membrane lipid asymmetry in bacteria.
Biological membranes comprise two layers (or leaflets) of fatty molecules, also known as lipids. They function as permeability barriers that define cellular boundaries. In Gram-negative bacteria, such as Escherichia coli, two membranes surround the cell with the outer membrane particularly good at preventing the entry of toxic substances. Therefore, only a few antibiotics are effective against infections caused by this class of bacteria. Our defences against Gram-negative bacterial infections are dwindling due to the rapid emergence of bacteria resistant to effective drugs. There is thus an ever urgent need to develop new classes of antibiotics to combat drug-resistant bacteria. As the outer membrane keeps many antibiotics out of the cell, an interesting approach to overcome drug resistance is to employ strategies that disrupt processes that are important for building and maintain a stable outer membrane. Here at NUS, scientists are gaining a better understanding of how the outer membrane is assembled, and learning how to disrupt it.
The outer membrane of Gram-negative bacteria has an uneven, or asymmetric, distribution of lipids in its two leaflets. The outer and inner leaflets of the membrane comprise two different forms of lipids, lipopolysaccharides and phospholipids, respectively. This unique lipid asymmetry is critical for the outer membrane to function as a permeability barrier. Therefore, understanding how outer membrane lipid asymmetry is established and maintained may provide useful insights towards designing novel approaches to combat drug-resistant bacteria. It has been discovered previously that a system known as the OmpC-Mla pathway is involved in maintaining outer membrane lipid asymmetry.This system includes a group of proteins (known as a complex) that functions as a transporter at the inner membrane. This transporter uses ATP hydrolysis (a biological process to convert chemical stored energy to mechanical movements) to power lipid movement at the membrane. What the transporter looks like and how it functions at the molecular level are not known.
Prof CHNG Shu Sin and graduate students THONG Shuhua and Bilge ERCAN from the Department of Chemistry, NUS have now characterised the organisation and function of this inner membrane transporter, which forms part of the OmpC-Mla system in Escherichia coli. The transporter comprises four proteins, including the core components, MlaF and MlaE, and two auxiliary proteins, MlaD and MlaB. It is demonstrated that six copies of MlaD form a highly stable complex that has strong affinity for phospholipid substrates. Furthermore, MlaB is required for both the assembly and activity of the transporter. This work provides hints on how the MlaFEDB complex may use ATP hydrolysis to drive overall lipid movement from the outer to the inner membrane, and sets the stage for future mechanistic questions to be addressed.
Going forward, the NUS team will directly establish the ability of the MlaFEDB complex in transporting lipids so as to enhance understanding of how ATP hydrolysis at the inner membrane can influence lipid asymmetry at the outer membrane. In the long term, their work, together with structural information at the molecular level, can provide important insights into how Gram-negative bacteria maintain outer membrane function.
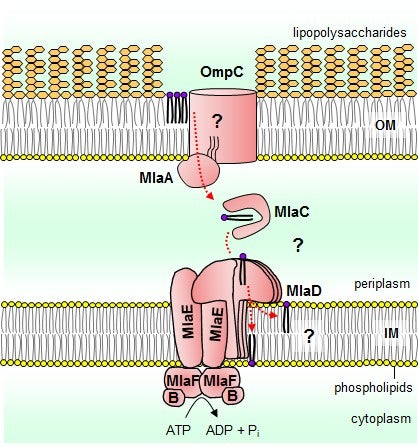
A schematic diagramme of the Gram-negative cell envelope and the function of the OmpC-Mla pathway in maintaining lipid asymmetry. The OmpC-MlaA complex may function to remove phospholipids from the outer leaflet of the outer membrane (OM), which are then transported via MlaC back to the MlaFEDB complex for insertion into, or transport across, the inner membrane (IM), at the expense of ATP. [Image credit: Chng Shu Sin]
Reference
Thong SH; Ercan B; Torta F; Fong ZY; Wong HYA; Wenk MR; Chng SS*, “Defining key roles for auxiliary proteins in an ABC transporter that maintains bacterial outer membrane lipid asymmetry” eLife 2016;5:e19042 DOI: 10.7554/eLife.19042 Published: 2016
Link for the paper: https://elifesciences.org/content/5/e19042 (open access).


