Deep tissue imaging and drug delivery
LIU Xiaogang ((Group Leader, Chemistry) ) April 28, 201728 Apr 2017. NUS scientists have developed a simple yet versatile nanoparticle system for deep tissue imaging and drug delivery.
Luminescent nanoparticles are an important group of materials for biomedical applications. These nanoparticles can be designed to attach themselves to specific biomolecules. This provides a simple way to identify and detect these biomolecules. The biomolecules could be proteins or antibodies in samples. This information allows medical professionals to diagnose and monitor patient conditions, deciding which therapies would work best for them. Researchers have developed a way to produce highly stable luminescent nanoparticles suitable for biological environments. They achieved this by modifying the surface condition of small particles. These biocompatible nanoparticles are suitable as probes for deep tissue imaging and therapeutic applications. Their method also overcomes the problem of nanoparticles tending to cluster together.
A research team led by Prof LIU Xiaogang in the Department of Chemistry and Prof Angelo Homayoun ALL from the Department of Orthopedic Surgery at NUS, together with researchers from the Agency for Science, Technology and Research (A*STAR), Nanjing Tech University (China), Molecular Imaging & Therapy Branch, National Cancer Centre (Korea) and Nanyang Technological University (Singapore) have designed and developed DNA-modified gold nanoparticles for use as nanoprobes. These nanoparticles have improved stability in a biological setting. The DNA strands bound on the surface of the nanoparticles allow them to retain their biological functionality. This means that the nanoparticle can be used for target strand hybridisation and molecular recognition.
There are many surface functionalisation strategies available to equip upconversion nanoparticles with different types of polymers, biomolecules, and proteins for specific applications. However, most of these strategies have limitations. They often produce nanoparticles with limited biocompatibility, specificity, functionality, and dispersibility, particularly in a high-salt physiological environment. Some of the processes are highly complex and it could be difficult to produce the nanoparticles in a scalable manner. This is one of the factors hindering a more widespread use of nanoparticles for biomedical applications.
The researchers found that DNA strands, when linked to small gold-based upconversion nanoparticles, showed improved stability and enhanced biological functions. The nanoparticles also did not exhibit problems, which usually occur when combining biological elements with upconversion nanoparticles. These include particle agglomeration, non-specific binding and biomolecule deactivation. More importantly, the optical interaction between gold and the upconversion nanoparticles provides the resulting nanoconjugates with a single-band near infrared (NIR) emission and photothermal effect. The photothermal effect is a phenomenon that happens when a material excited by light produces heat or thermal energy. These functionalities of the nanoconjugates are particularly useful for deep-tissue imaging and guided therapy treatment.
The DNA strand attached to the nanoparticle can also serve a drug delivery function. It can carry many types of DNA-binding drug molecules. As it is highly sensitive to fluctuations in temperature, the delivery of the drug molecules can be precisely controlled using the photothermal effect.
The ability to emit single-band NIR-to-NIR luminescence and deliver drug molecules on demand, together with high stability in a biological environment, makes these new upconversion probes a promising candidate for deep-tissue imaging and drug release applications. This work may provide a new direction for research into better understanding of luminescent probes for biomedical applications.
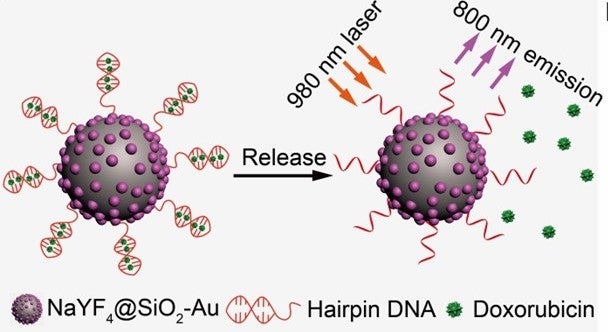
Figure shows design of hairpin DNA-functionalised NaYF4@SiO2-Au nanoparticles for photothermal drug release. Schematic illustration of doxorubicin drug release from DNA-modified nanoconjugates, triggered by a photon upconversion process. [Image credit: Sanyang HAN]
Reference
Han S; Samanta A; Xie X; Huang L*; Peng J; Park SJ; Teh DBL; Choi Y; Chang Y-T; All AH*; Yang Y; Xing B; Liu X*, “Gold and hairpin DNA functionalization of upconversion nanocrystals for imaging and in vivo drug delivery” ADVANCED MATERIALS DOI: 10.1002/adma.201700244 Published: 2017.


