Synthetic peptides as promising tuberculosis therapeutics
Rachel EE ((Group Leader, Pharmacy) ) January 06, 20176 Jan 2017. NUS pharmaceutical scientists have developed new antimicrobial peptides that are effective against multidrug-resistant tuberculosis.
Tuberculosis (TB) now ranks alongside HIV as the leading cause of death from an infectious disease worldwide, with 9.6 million new cases and 1.5 million deaths in 2014. The emergence of multidrug-resistant (MDR) TB has increased demand for new antibiotics with novel mechanisms of action, yet, paradoxically, antibiotic pipelines are shrinking.Antimicrobial peptides (AMPs) have gained considerable interest as a new source of antibiotics due to their broad-spectrum and rapid bactericidal activity. However, the transition of AMPs from bench to bedside has largely been hindered by their susceptibility to enzymes in biological fluids and degradation by bacterial proteases, resulting in short half-lives and loss of antimicrobial properties.
To address this issue, researchers from NUS, A*STAR and Imperial College London have teamed up to develop more stable and potent synthetic peptides for application in TB therapy. The interdisciplinary team, led by Prof Rachel EE Pui Lai from the Department of Pharmacy, NUS, and scientists from the Institute of Bioengineering and Nanotechnology and the Department of Medicine, Imperial College London, designed and evaluated a series of unnatural (not naturally encoded or found in the genetic code of any organisms) amino acid modified peptides against drug-susceptible and MDR clinical isolates of Mycobacterium tuberculosis.
While all modified peptides were more stable to protease degradation, the all D-amino acid peptide proved most effective when tested against a panel of mycobacterial strains. This analogue effectively reduced intracellular MDR-TB burden and permeabilised the mycobacterial membrane. This is the first study which systematically evaluates the impact of various stability-enhancing modifications on the anti-mycobacterial activity of synthetic AMPs.
These findings reiterate the importance of unnatural amino acid modifications in improving protease stability while highlighting their applicability in designing AMPs with enhanced mycobacterial selectivity. Looking ahead, the team plans to evaluate the clinical potential of the optimised lead compound in vivo using animal models of TB infection.
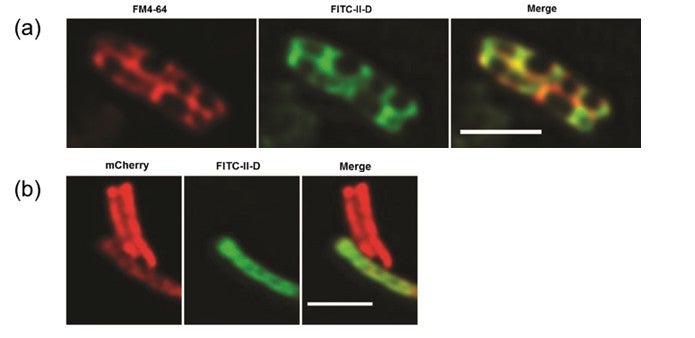
Figure shows fluorescently-labelled antimicrobial peptides, FITC-II-D (a) co-localising with a mycobacterial membrane dye, FM4-64, and (b) entering into red mCherry-labelled live attenuated strain of Mycobacterium bovis. Scale bar = 2mm.
This research was supported by the Singapore Ministry of Health’s National Medical Research Council under its Individual Research Grant (NMRC/1298/2011).
Reference
Khara JS; Priestman M; Uhia I; Hamilton MS; Krishnan N; Wang Y; Yang YY; Langford PR; Newton SM; Robertson BD; Ee PLR*, “Unnatural amino acid analogues of membrane-active helical peptides with anti-mycobacterial activity and improved stability” JOURNAL OF ANTIMICROBIAL CHEMOTHERAPY Volume: 71 Issue: 8 Pages: 2181-2191 DOI: 10.1093/jac/dkw107 Published: 2016


