New strategy towards stable acene dimers
CHI Chunyan (Group Leader, Chemistry) () May 07, 201607 May 2016 Scientists in NUS developed a new strategy to prepare acene dimers which have extremely high stability in ambient light and air conditions.
Acenes have attracted long-term interest because of their unique electronic structure and physical properties, and promising applications for organic electronics. However, the intrinsic high reactivity arising from their high-lying highest occupied molecular orbital energy levels have limited their practical applications. A team led by Prof CHI Chunyan from the Department of Chemistry in NUS has developed a new strategy to stabilise reactive acenes by fusion of an anti-aromatic pentalene unit onto the zig-zag edges of two acene units to form a Z-shaped acene dimer, which turned out to be extremely stable due to intramolecular donor-acceptor interaction and kinetic blocking. The half-life time of a tetracene dimer is about 54 days in solution under ambient light and air conditions, which is much more stable than the other reported analogues with half-life times of a few hours under the same conditions.
Acene-based materials have promising applications for organic electronics but the major constraint comes from their poor stability. The strategy developed in this work provides important guidance for the design of stable acene-based materials. The obtained acene dimers can also be used as stable semiconductors for organic electronics.
This new strategy can be used to make stable acenes, heteroacenes, and perifused acenes in the future, which are novel and promising materials for organic electronics, phototronics and spintronics.
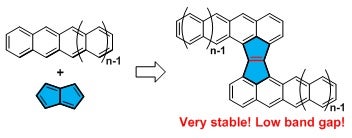
Figure shows the strategy towards stable acene dimers [Image credit: Dai GAOLE].
Reference
Dai G, Chang J, Luo J, Dong S, Aratani N, Zheng B, Huang KW, Yamada H, Chi C. “Z-Shaped Pentaleno- Acene Dimers with High Stability and Low Band Gap” Angew. Chem. Int. Ed. 55 (2016) 2693.


