The critical challenge addressed by the team is that current treatment options for PAH are administered as intravenous infusions or inhalation solutions. Various controlled release inhalation treatments, ranging from liposomes, biodegradable nanoparticles and microparticles, co-precipitates and complexations with cyclodextrins, have been explored. The most effective treatment requires the patient to remember to inhale the medicine six to nine times daily.
The innovative research reported by the team of pharmacists and engineers is the development of novel polymeric microspheres for inhalation to reduce dosing frequency and improve medication compliance. These microspheres are designed with release modifiers to reside in the lung, which is the site for the drug to be released slowly and consistently. The development of inhalation formulations accomplished thus far will need to be further optimised and subjected to in vivo and clinical studies.
This study was conducted by Dr Aparna SAIGAL during her Ph.D. scholarship with the generous support of the NUS Industrial Postgraduate Programme, under the guidance of Profs Wai Kiong NG, Reginald TAN and Sui Yung CHAN who are experts on the pharmaceutical formulation of active pharmaceutical ingredients (APIs) for the commercialisation of new chemical entities (NCE) as well as the refinement and repurposing of existing APIs to maximise their bioavailability and stability.
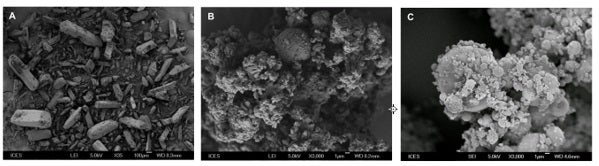
Figures show the scanning electron microscopy images of (A) Unprocessed drug particles, (B) Spray-dried drug particles and (C) Microspheres containing drug particles. [Image credit: Saigal A]
References
1. Saigal A, Ng WK, Tan BH, Chan SY. “Controlled Release Inhalable Polymeric Microspheres for Treatment of Pulmonary Arterial Hypertension.” Curr Pharm Des. 21 (2015) 5868.
2. Saigal A, Ng WK, Tan BH, Chan SY. “Development of Controlled Release Inhalable Polymeric Microspheres for Treatment of Pulmonary Hypertension.” Int J Pharm. 450 (2013) 114.


