A tale of two lipids for drug release
CHAN Lai Wah (Group Leader, Pharmacy) () August 14, 201514 August 2015 NUS scientists investigated the solid state characteristics of ibuprofen-loaded spray-congealed solid lipid microparticles (SLMs) and impact of drug-matrix miscibility on drug release.
A team led by Prof CHAN Lai Wah from the Department of Pharmacy in NUS with her graduate student Ms Priscilla Wong has discovered suitable lipids for spray congealing to produce solid lipid microparticles (SLMs) with different release characteristics. The relatively new one-step, solvent-free spray congealing process is useful for producing dense and highly spherical microparticles for controlled drug release and other applications, such as protection of moisture-sensitive drugs. As this process involves rapid cooling, one drawback is that lipid matrices and drug may crystallize in different polymorphic forms, affecting drug release and drug stability. Polymeric additives, namely polyvinyl-2-pyrrolidone-vinyl-acetate (PVP/VA) and ethylcellulose (EC), are suitable release modifying agents for these lipid-based formulations. The findings are useful in the development of specialised drug delivery systems.
In this study, a wide range of lipids that include cetyl alcohol (CA) and stearic acid (SA), and ibuprofen (IBU), a non-steroidal anti-inflammatory drug, were employed. IBU was less miscible with CA than SA, forming a solid dispersion and solid solution respectively at 20 %, w/w IBU. When these lipid formulations were spray-congealed, the crystalline drug was converted to its amorphous form while the two lipids retained their stable polymorphic form. CA was found to release IBU faster than SA. PVP/VA and EC affected the crystalline structure of CA but had no effect on SA. Nevertheless, both polymeric additives increased the rate of drug release to different extent.
This study made use of a combination of spectroscopic and calorimetric techniques to characterize lipid-based formulations and explain their drug release profiles (see Figure). Using lipid casts, differential scanning calorimetry (DSC) was used to determine drug-lipid solid miscibility. Nuclear magnetic resonance (NMR) and Fourier-transformed infrared (FTIR) spectroscopy were used to establish the types of molecular interaction between lipids and polymers. Combined with X-ray diffractometry (XRD), it was found that polymers could expand the unit cells of certain lipids via hydrogen bonding. Drug-matrix miscibility was also found to be an important factor governing the drug release profile of lipid-based formulations.
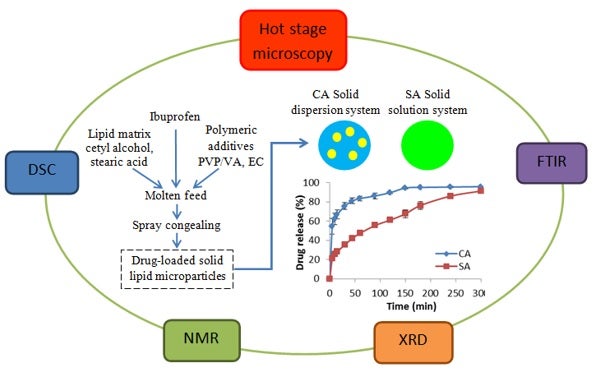
SLMs were produced from different lipids (CA or SA), polymeric additives (PVP/VA or EC) and drug (ibuprofen) by spray congealing. Using a combination of calorimetric (DSC, hot stage microscopy) and spectroscopic (NMR, FTIR, XRD) techniques, drug-matrix miscibility and polymorphic changes were determined. Dissolution studies were performed to investigate drug release from the different formulations. [Image credit: Priscilla Wong]
References
1. Wong CHP, Heng PWS, Chan LW. “Determination of solid state characteristics of spray-congealed ibuprofen solid lipid microparticles and their impact on sustaining drug release.” Molecular Pharmaceutics. 12, no. 5 (2015) 1592.
2. Rodriguez L, Albertini B, Passerini N, Cavallari C, Giovannelli L. “Hot air coating technique as a novel method to produce microparticles.” Drug Dev Ind Pharm. 30 ( 2004) 913.
3. Westesen K, Bunjes H, Koch MHJ. “Physicochemical characterization of lipid nanoparticles and evaluation of their drug loading capacity and sustained release potential.” J Control Release. 48 (1997) 223.


