The later definition of temperature may result in a negative value for the temperature T, if the entropy of the system decreases with the energy of the system. The negative temperature is actually hotter than the positive temperature, not colder, because it has more energy [see Figure]. Indeed, the experimental results of Purcell and Pound (1951) were interpreted this way. However, this landmark result and conclusion is currently disputed. The source of the disagreement is on which version of the definition of entropy one relies. According to them, the entropy originally proposed by Boltzmann was wrong, and the correct one should be based on the phase space volume less or equal to a given value of energy E. This later definition is due to Josiah W Gibbs.
Prof WANG Jian-Sheng from the Department of Physics in NUS and Prof Robert SWENDSEN from Carnegie Mellon University disagreed. They argued that thermodynamics applies only to large systems and Boltzmann’s definition of entropy based on probability satisfies all the requirements of thermodynamics as explicitly stated in Herbert Callen’s popular textbook. Other authors also pointed out the fault of Dunkel’s and Hilbert’s argument.
Prof Wang has been teaching an advanced statistical mechanics course for many years. Therefore he felt that it is necessary to set things straight. This resulted in a paper accepted by American Journal of Physics.
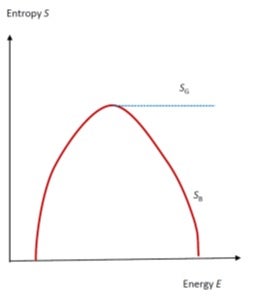
This figures shows the entropy versus energy curve. A decrease of entropy with energy results in negative temperature, but Gibbs’s definition gives a flat curve on the high energy side. [Wang JS]
References
1. EM Purcell, RV Pound. “A nuclear spin system at negative temperature” Phys Rev 81 (1951) 278.
2. J Dunkel, S Hilbert. “Consistent thermostatistics forbids negative absolute temperatures” Nature Phys 10 (2014) 61
3. Swendsen RH, Wang JS. “Negative temperature and the definition of entropy” American Journal of Physics http://arxiv.org/abs/1410.4619


