Protein dynamics determinant for ALS
Song Jianxing (Group Leader, Biological Sciences) () April 13, 201513 Apr 2015 NUS scientists deciphered that protein dynamics are a central determinant for whether a small molecule might act as a drug or poison to ALS patients.
16 Eph receptors constitute the largest family of receptor tyrosine kinases, controlling various physiological and pathological processes. Most strikingly, EphA4 was recently identified as the only target for designing drugs to treat amyotrophic lateral sclerosis (ALS). EphA4 inhibitors could enhance the survival rate of ALS patients but activators might be poisonous.
For more than 15 years, Prof SONG Jianxing from the Department of Biological Sciences in NUS has been working on structural and dynamic mechanisms, as well as drug design for Eph signaling. Previously, his team found that two small molecules bind the same EphA4 channel at almost equivalent affinities but mysteriously trigger opposite signaling outputs: one activated but another inhibited. Now his team decoded that the different outputs are unexpectedly achieved by oppositely modulating EphA4 dynamics.
Despite its first description in 1869, so far there is no primary therapy for ALS. The recent discovery that inhibition of EphA4 could rescue ALS-phenotype opens up a valueless potential to design drugs to treat ALS. Indeed, small molecules Prof Song’s team previously characterized were shown to rescue ALS animal models. The new results from Prof. Song’ team now established a dynamic principle which has to be satisfied in design of drugs to increase the survival of ALS patients by inhibiting EphA4. The drugs must enhance, or at least not suppress, EphA4 dynamics particularly on µs-ms time scale.
To comprehensively fight ALS, Prof Song’s team is currently focused on: 1) the mechanism by which aggregation of ALS-causing proteins (such as SOD1 and TDP-43) gains cellular toxicity; 2) the mechanism of oxidative stress initiating neurodegeneration; and 3) design/discovery of EphA4 inhibitors. It will be very promising to identify EphA4 inhibitors from plants. For example, compounds from green tea have been shown to inhibit EphA4. On the other hand, for ALS patients, it is also critical to avoid to intake EphA4 activators which may decrease the survival rate.
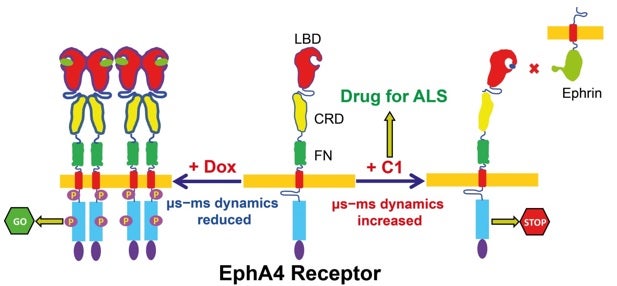
The image shows the proposed mechanism for activating/inhibiting the membrane-anchored EphA4 receptor by two small molecules. Upon binding doxazosin, μs−ms dynamics dramatically reduced, which triggers the ectodomains dimerization, followed by further clustering. As such, the EphA4-mediated signaling will be activated even without the ephrin-binding. In contrast, upon binding C1, μs−ms dynamics significantly increased, thus disfavoring the dimerization. Furthermore, as the ligand binding channel is occupied by C1, the binding of ephrin to EphA4 is physically inhibited. Together, the EphA4 receptor remains inactivated. [Image credit: Song Jianxing]
References
1. Qin H, Lim LZ, Song J. “Dynamic Principle for Designing Antagonistic/Agonistic Molecules for EphA4 Receptor, the Only Known ALS Modifier.” ACS Chem Biol. 10 (2015) 372.
2. Qin H, Shi J, Noberini R, Pasquale EB, Song J. “Crystal structure and NMR binding reveal that two small molecule antagonists target the high affinity ephrin-binding channel of the EphA4 receptor.” J Biol Chem 283 (2008) 29473.


