This effect is related to the bursting of popcorn kernels at high temperatures. It is termed the “photosalient effect” and is one pathway for the conversion of light into mechanical motion. The discovery paves the way to exploit bond-forming photochemical reactions in solids for the photosalient effect.
The team hopes to eventually develop new materials that could convert solar energy effectively into mechanical energy. This will enable the development of actuators and mechanical devices that bend, change shape, or store mechanical energy from sunlight.
Their findings were published as the cover story in the English version of German scientific journal Angewandte Chemie International Edition.
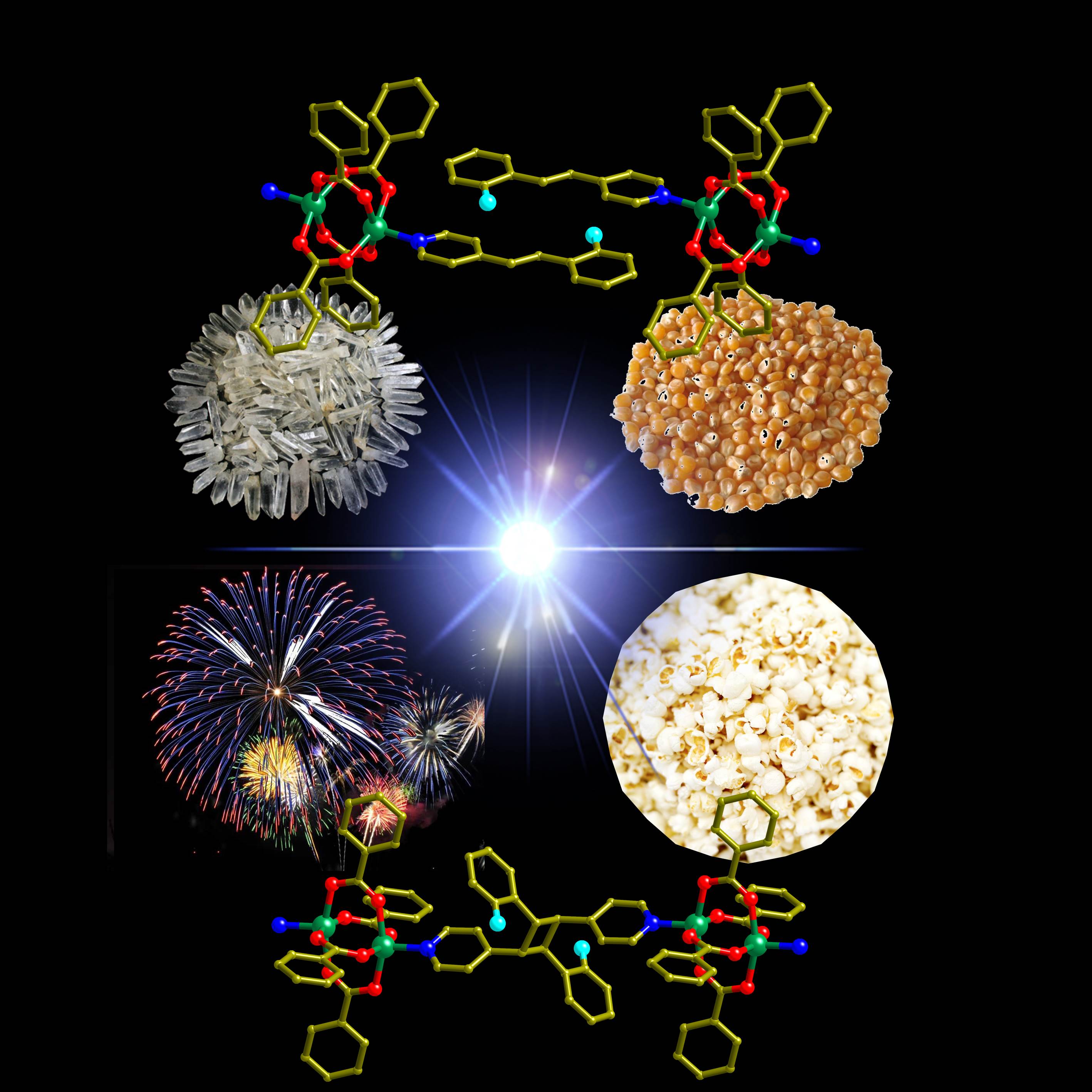
A schematic diagram showing the popping nature of the crystals under UV light, a property that is very similar to the popping of corns on a hot plate. (Image credit: National University of Singapore)


