Making/breaking C-C bonds in single crystals!
Jagadese J. VITTAL (Group Leader, Chemistry) () May 13, 201413 May 2014. Single Crystal to Single Crystal Bond can be made broken.
The cyclobutane ring can easily be made by solid-state [2+2] cycloaddition reaction. But can this be cleaved? Is it possible to make and break the C-C bond reversible? Can this be achieved in a single-crystal to single-crystal manner? Will this be accompanied by change in properties like photoluminescence? If so the applications for these types of systems are eminent in molecular switching and data storage.
It is very rare for single crystals to sustain its nature in two consecutive structural transformations. This study has demonstrated a simple but interesting metal organic compound that exhibits photochemical formation and thermal cleavage of cyclobutane ring in cyclic manner and with an efficiency of 0.848. The SCSC processes involving photodimerization, dehydration and reversible thermal cleavage allowed Professor Jagadese J. VITTAL’s group to follow the structural transformations unequivocally by X-ray crystallographic analysis. Of the two polymorphic form discovered, the monoclinic form is not able to withstand its single crystallinity during photodimerization reaction but the orthorhombic form, the thermodynamic product us able to retain the single crystallinity (see Figure). Polymorphs are known to show different physical properties and chemical reactivities. Although retention of single crystals during a reaction solely depends on the experimental skills of the researchers and advancement in the instrumentation, this work reveals that retention of single crystals is strongly associated with the packing of molecules, which are controlled by kinetics and thermodynamics, and yet another property of polymorphism. This indicates that single crystal nature during a chemical reaction can be controlled by packing which is dictated by thermodynamics and kinetics, is an unexplored area in crystal engineering. Our preliminary results on the photo-emissive properties of the photo- and thermal-products demonstrated that this system can be extended to design materials for practical applications in optical recording, photo-switches and sensors.
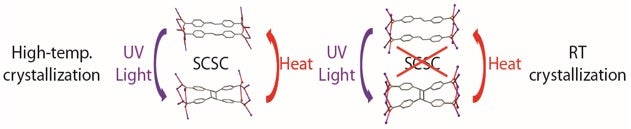
Image shows the Reversible Single-Crystal-to-Single-Crystal Photochemical Formation [Image credit: Jagadese J. VITTAL]
Reference
GK Kole, T Kojima, M Kawano, JJ Vittal. “Reversible Single-Crystal-to-Single-Crystal Photochemical Formation and Thermal Cleavage of a Cyclobutane Ring.” Angewandte Chemie International Edition 53 (2014) 2143.


