Distorted polymers make good sensors
Jagadese J. VITTAL (Group Leader, Chemistry) () May 14, 201414 May 2014. A distortional isomerism has been discovered in coordination polymers that leads to enhance sensitivity to certain chemical reagents.
Distortional isomerism is a new class of supramolecular isomerism. Distortional isomerism is a phenomenon in which the molecule exists in different forms with changes in their bond lengths. This has been found to be arise from crystallographic disorder in most cases, However it can also exist in copper compounds as a result of their plasticity. Here the team led by Professor Jagadese J. VITTAL from the Department of Chemistry in NUS has discovered distortional isomerism in a class of two-dimensional polyrotaxane coordination polymers. These polymers contain large rings spaced by long ligands (see Figure). The distortion involves displacements in the relative positions of the wheel in neighboring entangled axles. As a result four different isomers can be obtained, of which two have entangled polyrotaxane structures. In one of them, one pair of C=C bonds in the spacer ligand is well-aligned with the pair in the adjacent spacer. These can then undergo a UV-light-induced [2+2] cycloaddition reaction. However the other isomer is photo-inert. Apart from differences in photochemical reactivity, they also have different sensing efficiency for aromatic nitro compounds. Both the isomers show selective photoluminescence quenching for the Brady’s reagent (2,4-dinitrophenylhydrazine), which is an explosive, and other nitro compounds.
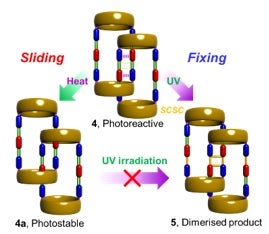
Image shows the distortional isomerism in polyrotaxane coordination polymers [Image credit: Jagadese J. VITTAL]
Reference
Park IH, Medishetty R, Kim JY, Lee SS, Vittal JJ. “Distortional Supramolecular Isomers of Polyrotaxane Coordination Polymers: Photoreactivity and Sensing of Nitro Compounds.” Angewandte Chemie International Edition 53 (2014) 22.


