A new concept for single crystal materials
Jagadese J. VITTAL (Group Leader, Chemistry) () May 13, 201413 May 2014. Single crystals of organic polymers or the metal complexes of organic polymeric ligands are not known since they are difficult to crystallize by traditional methods.
Vittal’s group reported an organic polymer comprising cyclobutane rings and a coordination polymer blend together inside this 3D structure obtained in a photochemical dimerization reaction. The organic polymer can be depolymerized by the cleavage of cyclobutane rings in an SCSC manner. Such highly mono-crystalline metal complexes of organic polymers were hitherto inaccessible for materials researchers.
Organic polymers are usually amorphous or possess very low crystallinity. The metal complexes of organic polymeric ligands are also difficult to crystallize by traditional methods due to their poor solubilities and therefore, their three-dimensional structures could not be determined by single crystal X-ray crystallography due to the lack of single crystals. Here we report the crystal structure of a one-dimensional Zn(II) coordination polymer fused with an organic polymer ligand made in situ by [2+2] cycloaddition reaction of a six-fold metal-organic framework. It is also shown that this organic polymer ligand can be depolymerised in a single-crystal to single-crystal (SCSC) fashion by heating. One can potentially extend this strategy to make a range of monocrystalline metal organo-polymeric complexes and metal-organic organo-polymeric hybrid materials. Such highly monocrystalline metal complexes of organic polymers were hitherto inaccessible for materials researchers.
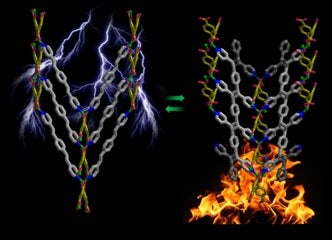
Image shows single crystals of a coordination polymer containing an organic polymer ligand [Image credit: Jagadese J. VITTAL]
Reference
Park IH, Chanthapally A, Zhang Z, Lee SS, Zaworotko MJ, Vittal JJ. “Metal-Organic Organo-Polymeric Hybrid Framework by Reversible [2+2] Cycloaddition Reaction.” Angewandte Chemie International Edition 53 (2014) 414.


