Role of oxygen in hydrogen formation
CHUAH Gaik Khuan ((Group Leader, Chemistry)) March 02, 20172 Mar 2017. NUS chemists have discovered that oxygen gas is vital for the alcohol dehydrogenation process at low temperatures.
Hydrogen gas is a clean source of energy as the end-product of its combustion is water. As hydrogen gas does not occur naturally in sufficiently large quantities, there is a lot of research interest to produce it from different sources. Alcohols can be a fuel source for hydrogen gas. However, obtaining hydrogen gas from an alcohol to be used as fuel is an energetically uphill process at low temperatures. High temperatures of more than 200°C are typically required for such reactions, which in themselves are energy expending. A team led by Prof CHUAH Gaik Khuan from the Department of Chemistry, NUS together with her colleagues showed that in a closed system, the conversion of benzyl alcohol to hydrogen and benzaldehyde can occur at a much lower temperature of 120°C but this is only possible in the presence of some oxygen. Even then, only a small amount of alcohol reacts to form hydrogen (less than 2.5% of the alcohol) with the bulk forming water.
In recent years, the acceptor-less or oxidant-free dehydrogenation of alcohols has attracted much research interest. Hydrogen generated from bio-alcohols can be utilised in fuel cells as a carbon-neutral means of locomotion. However, oxidant-free dehydrogenation reactions being energetically uphill, require some form of energy input to kick-start the reactions. Although there are reports suggesting that alcohol dehydrogenation reactions may be possible at low temperatures by using specially designed catalysts, the research team has shown that hydrogen cannot be formed fully from alcohol in such closed systems.
Thermodynamics principles predict that it is not possible to form significant amounts of hydrogen from alcohols at low temperatures in a closed system, even with the best catalyst. This is in agreement with their experimental results. In the presence of low levels of oxygen, water is preferentially formed from the reaction. As this reaction is highly exothermic (chemical reaction that releases energy), this energy can be used to drive the hydrogen evolution reaction, but this remains limited to a few percent. In open systems, where there is enough oxygen present, water is preferentially formed with little or no hydrogen created.
The research team has developed an active silver on alumina catalyst from this work and plan to further investigate the photoactivity of this catalyst. With photocatalysis, energy from the sun can be used to break the chemical bonds in the alcohol molecule to form hydrogen.
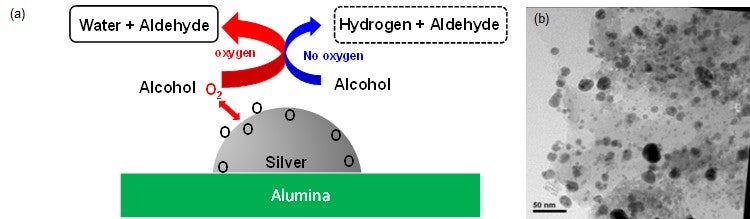
Figures show (a) the removal of hydrogen from alcohol in the presence of oxygen and without oxygen, (b) transmission electron micrograph of catalyst showing silver particles on alumina.
Reference
Liu HH; Tan H-R; Tok ES; Jaenicke S*; Chuah GK*, “Dehydrogenation of Alcohols over Alumina-Supported Silver Catalysts: The Role of Oxygen in Hydrogen Formation” CHEMCATCHEM Volume: 8 Issue: 5 Pages: 968-975 DOI: 10.1002/cctc.201501200 Published: 2016


