Artificial photosynthesis to produce hydrogen peroxide
June 14, 2024National University of Singapore (NUS) chemists have developed hexavalent photocatalytic covalent organic frameworks (COFs) which mimic natural photosynthesis for the production of hydrogen peroxide (H2O2), an important industrial chemical.
The conventional method of H2O2 production involves using anthraquinone as a catalyst to convert air and hydrogen into H2O2. However, this process requires substantial energy, costly noble metal catalysts, high-pressure hydrogen gas and hazardous solvents. Artificial photosynthesis of H2O2, resembling the natural photosynthesis process with the use of sunlight as an energy source and abundant water and air as feedstocks, presents a sustainable and promising alternative to the conventional anthraquinone process. However, such an artificial system faces three key challenges: (1) insufficient charge carrier generation and fast charge recombination, which lowers the efficiency; (2) limited number of available catalytic sites, which results in low productivity; and (3) lack of efficient delivery of charges and reactants to the catalytic sites, which causes sluggish reaction kinetics.
The research team, led by Professor Donglin JIANG from the Department of Chemistry at NUS has conceived a new strategy that develops hexavalent photocatalytic COFs for efficient photosynthesis via systematic design of the π skeletons and pores. These COFs are porous, crystalline materials built from organic molecules linked together by strong covalent bonds. Their inherent flexibility makes them an ideal platform for constructing photocatalysts. The researchers created a new type of donor-alt-acceptor framework photocatalysts that, upon irradiation, are converted into catalytic scaffolds with dense catalytic sites for oxygen reduction and water oxidation. These photocatalysts possess spatially segregated donor and acceptor columns for holes and electrons separation to prevent charge recombination and enable rapid charge transport. Moreover, the pore walls of the photocatalytic COFs are engineered to be hydrophilic to facilitate water and dissolved oxygen to pass through the 1-dimensional channel to reach the catalytic sites via capillary effect.
The research findings were published in the journal Nature Synthesis.
Functioning as a photocatalyst for H2O2 production using only water, air and light, the COFs achieve impressive metrics: a production rate of 7.2 mmol g–1 h–1, an optimal apparent quantum yield of 18.0% and a solar-to-chemical conversion efficiency of 0.91% in bath reactors. Upon integration into flow reactors, they sustainably produce over 15 litres of pure H2O2 solution under ambient conditions, demonstrating operation stability over a two-week period.
Prof Jiang said, “This work embodies nearly two decades of our collective efforts in the field of COFs, culminating in the development of novel photocatalysts that effectively address two fundamental yet formidable challenges: the simultaneous and efficient delivery of charges and mass to catalytic sites. The breakthroughs presented herein signify a compelling and paradigm-shifting advancement in the realm of artificial photosynthesis.”
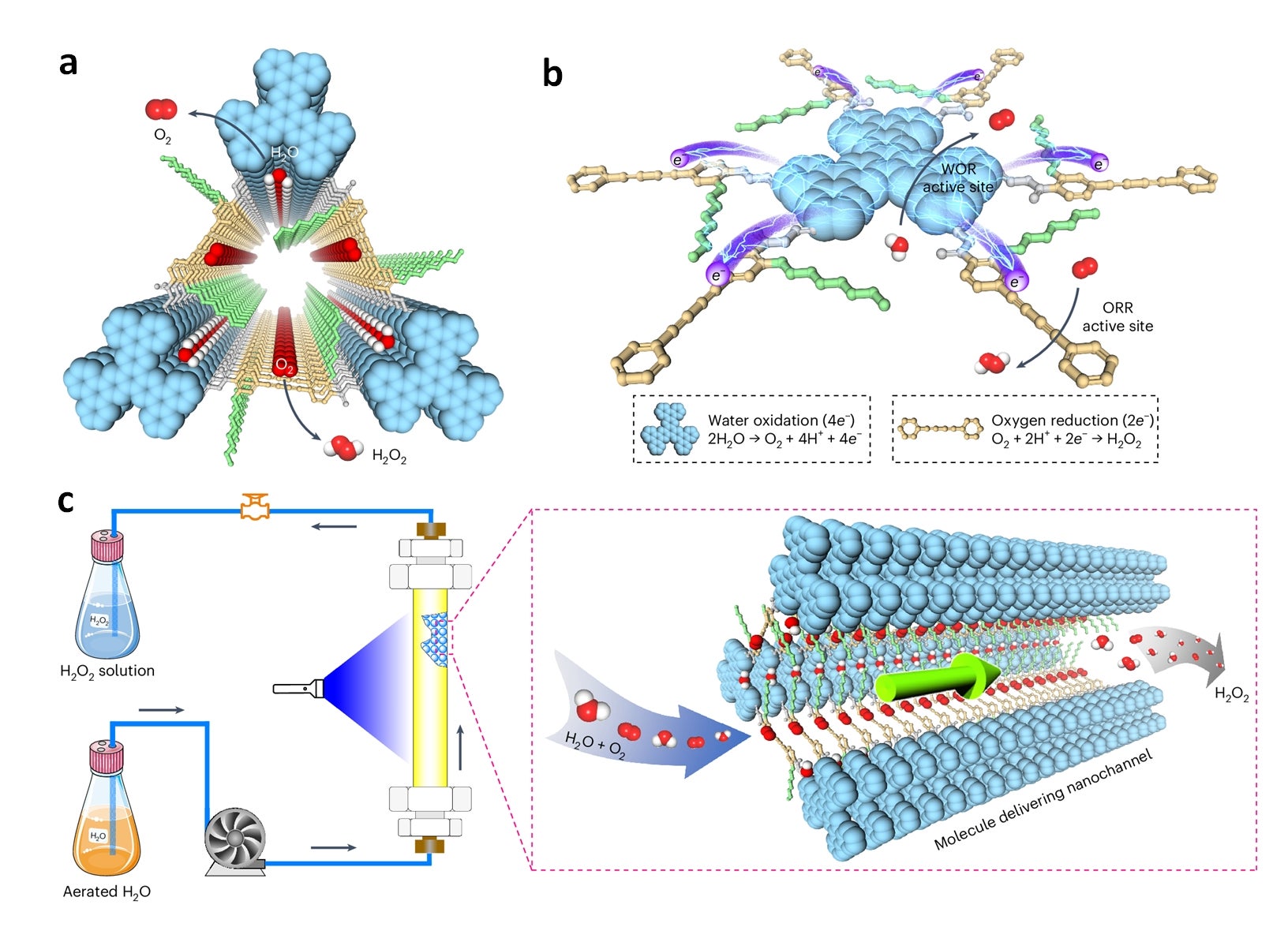
Figure shows the systematic design of the photocatalytic covalent organic frameworks (COFs) for manufacturing hydrogen peroxide (H2O2) using flow reactors. (a) The COF with segregated donor and acceptor zones along with specially designed water channels. (b) Under light, charges separate, with oxygen reduction occurring at one region, and water oxidation at another region. (c) Continuous production of H2O2 occurs in a flow reactor, where water and oxygen transform into H2O2 through the tiny channels of the photocatalyst. [Credit: Nature Synthesis]
Reference
Chen YZ; Liu RY; Guo YY; Wu G; Sum TC; Yang SW; Jiang DL*, “Hierarchical assembly of donor-acceptor covalent organic frameworks for photosynthesis of hydrogen peroxide from water and air” Nature Synthesis DOI: 10.1038/s44160-024-00542-4 Published: 2024.


