When are many-body effects significant?
March 22, 201822 Mar 2018. NUS computational chemists have developed a method that can quickly identify which interactions between groups of molecules, or between parts of a very large molecule, are small and may be ignored. This enables the interactions between molecules to be computed more efficiently and accurately.
Many-body effects, which refer to the collective behaviour of a large number of interacting constituents, are required for an accurate description of both the structure and dynamics of large chemical systems such as a protein molecule, or describing the properties of bulk polar solvents. Most often, however, these effects are simply ignored because there is a huge number of possible interactions between the various constituents and it is usually not obvious which of these would have a significant effect. Typically, when the many-body effects are ignored, approximations must be made in an attempt to account for them. On the other hand, having to compute all the possible many-body interactions in large chemical systems uses a huge amount of computational resources.
A team led by Prof Ryan BETTENS from the Department of Chemistry, NUS has developed a general method that can quickly identify a small set of trimer (three-body) and tetramer (four-body) interactions that are responsible for the vast majority of these higher body effects in large chemical systems. This is achieved by quickly and accurately determining the maximum possible interaction each individual trimer and tetramer can make to the overall interaction. If the maximum possible interaction for a trimer or tetramer is too small to make a significant contribution to the overall interaction energy in a large system, it is ignored. In this way, the number of computations required can be reduced by a few orders of magnitude and still produce highly accurate outcomes.
When working on the computation for many-body effects, the researchers also found two main causes for significant many-body interactions. First, many-body induction propagates in non-branching paths. This means that the interactions between the bodies happen in a chain-like manner, one after another. Second, linear arrangements of bodies promote the alignment of molecular polarity (charge dipole) which reinforces many-body interactions. As a result, molecules tend to have compact and extended linear arrangements. Compact arrangements are favoured because of the many short non-branching paths connecting the bodies. Extended linear arrangements are also preferred as they favour dipole alignment.
Prof Bettens said, “This study provides a rigorous explanation on how cooperative effects (synergistic interactions) provide enhanced stability in helices, making them one of the most common structures in biomolecules. Not only do these helices promote linear dipole alignment, but their chain-like structure is consistent with the way many-body induction propagates.”
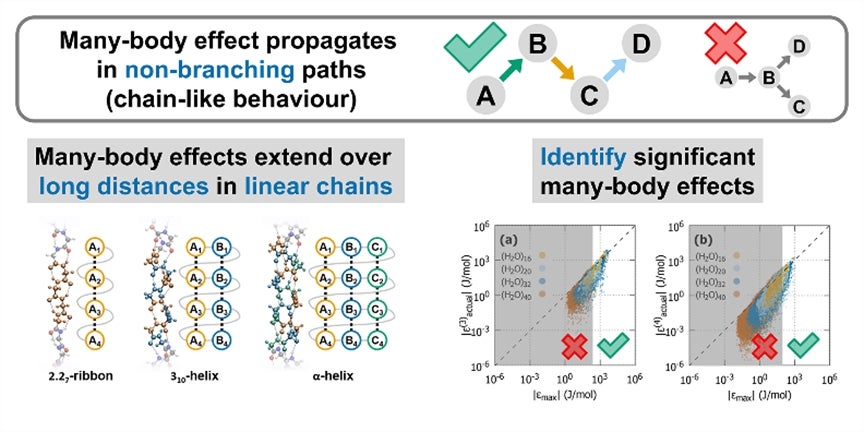
(Upper pane) Many-body effects occur most significantly through chains, not branches. (Lower left) The cooperative effects which are the most significant result in the enhanced stability of helices found in proteins. (Lower right) A simple energy criterion can be used to significantly reduce the computation effort required to obtain the many-body interactions. [Copyright 2016 American Chemical Society]
Reference
Ouyang JF; Bettens RPA*, “When are Many-Body Effects Significant?” JOURNAL OF CHEMICAL THEORY AND COMPUTATION Volume: 12 Issue: 12 Pages: 5860-5867 DOI: 10.1021/acs.jctc.6b00864 Published: 2016.


