Clar’s sextet rule finds new applications
WU Jishan (Group Leader, Chemistry) () May 10, 201410 May 2014. Clar’s aromatic sextet rule was demonstrated to be effective in predicting the biradical character of benzenoid polycylic hydrocarbons with a singlet biradical ground state.
The Clar’s aromatic sextet rule is known as a classic rule to evaluate the stability and reactivity of closed-shell polycyclic aromatic hydrocarbons (PAHs). A team led by Professor WU Jishan from the Deaprtment of Chemistry in NUS recently found that certain type of PAHs could have an open-shell biradical ground state. In this work, we demonstrated that Clar’s aromatic sextet rule can be further applied to the open-shell PAHs, that is to say, for benzenoid polycyclic hydrocarbons with the same chemical composition, the molecule with more aromatic sextet rings in the biradical resonance forms exhibits greater singlet biradical character. The extension provides importance guidance for the design of PAHs with tunable biradical character.
Open-shell PAHs have showed unique electronic, optical and magnetic activity and are different from the traditional closed-shell PAHs. They can be regarded as next generation molecular materials for organic electronics, spintronics, photonics, photovoltaics and energy storage devices. Our recent studies showed that it is of importance to control the biradical character to tune the physical properties and material applications. Our new discovery of the Clar’s aromatic sextet rule in singlet biradicaloids is very important for future tailored design of materials with tunable properties and functions. This work was published in Journal of the American Chemical Society and also highlighted in SYNFACTs.
The Clar’ Aromatic Sextet Rule has been widely used for the prediction of the reactivity and stability of PAHs with a closed-shell electronic configuration. Recent advances in open-shell biradicaloids have shown that the number of aromatic sextet rings played important role in determination of their ground states. In order to test the validity of this rule in singlet biradicaloids, two soluble and stable dibenzoheptazethrene isomers DBHZ1 and DBHZ2 were prepared and isolated in crystalline form (see Figure). These two molecules own different numbers of aromatic sextet rings in their respective biradical resonance forms and thus are expected to exhibit varied singlet biradical character. This assumption was verified by different experimental methods assisted by DFT calculations. DBHZ2 with more aromatic sextet rings in the biradical form was demonstrated to possess larger biradical character than DBHZ1, as a result, DBHZ2 exhibited an intense one-photon absorption in the near-infrared region and a large two-photon absorption cross-section.
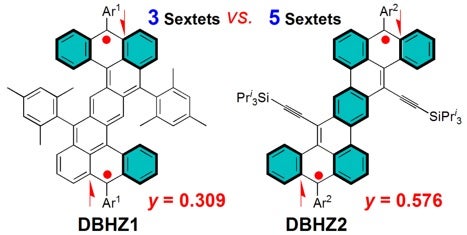
Image shows the singlet biradicaloids [Image credit: WU Jishan]
Reference
Sun Z, Lee S, Park K, Zhu X, Zhang W, Zeng B, Hu P, Zeng Z, Das S, Li Y, Chi C, Li R, Huang K, Ding J, Kim D, Wu J. “Dibenzoheptazethrene isomers with different biradical characters: an exercise of Clar’s aromatic sextet rule in singlet biradicaloids.” Journal of the American Chemical Society 135 (2013) 18229.


