Metabolic profiles of Alzheimer’s disease
Paul HO (Group Leader, Pharmacy) () August 12, 201512 Aug 2015 NUS professors studied the metabolic profiles of an amyloid beta-protein precursor (AβPP)-transfected CHO cells (CHO-AβPP695), an in-vitro model of Alzheimer’s disease.
Prof Paul HO and Eric CHAN from the Department of Pharmacy in NUS together with the graduate student Mr CHANG Kai Lun used the metabolomics approach to profile the AβPP695 CHO cells and its wildtype (see Figure). The team’s finding have indicated that the two cell types could be discriminated through their metabolic profiles as early as 24 hours after incubation, while the amyloid β42 (Aβ42), a hallmark of Alzheimer’s disease was upregulated in the CHO-AβPP695 cells only after 48 hours of incubation. These results were confirmed by further biochemical assays, indicating that mitochondrial dysfunction could happen prior to amyloid-β pathology. The observed 24-hour metabolic fluxes in the CHO-AβPP695 cells were associated with increased mitochondrial AβPP and reduced mitochondrial viabilities, which occurred before extracellular Aβ accumulation.
They have also investigated the therapeutic potential of peroxisome proliferator-activated receptor gamma (PPARγ) agonists, two anti-diabetic drugs namely rosiglitazone (RSG) and pioglitazone (PIO) for the treatment of Alzheimer’s disease, by employing the same approach to characterize the metabolic profiles of CHO-AβPP695 treated with RSG and PIO, with or without their respective receptor blockers. Treatment with PIO was found to reduce the perturbation of the discriminant metabolites in CHO-AβPP695 to a larger extent than treatment with RSG. They also attributed the PIO effects on the lowering of Aβ42, and restoration of mitochondrial activity to the respective PPARγ and PPARα agonism. In the study, PIO was demonstrated to be therapeutically superior to RSG. These findings will provide further insights into the early disease stages in this AβPP model, and support the advancement of PIO in Alzheimer’s disease therapy.
Takeda Pharmaceutical Company is recruiting 5800 subjects for a 5-year phase III clinical trial to look at the therapeutic potential of PIO in Alzheimer’s disease. The information acquired in this study could prove to be useful for the research scientists and clinicians involved in the clinical trial.
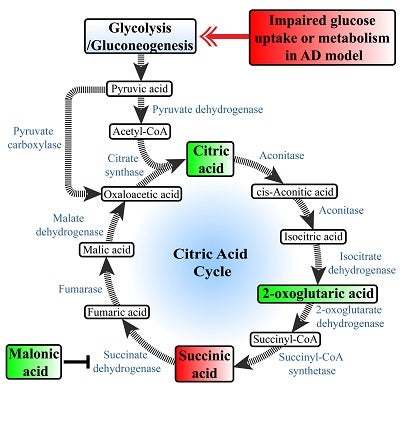
Graphical illustration of fluxes in APP-perturbed metabolites associated with citric acid cycle. Metabolites in green boxes were detected at higher level and metabolites in red boxes were shown to be lower in CHO-AβPP695 cells, an in-vitro Alzheimer’s disease model [Reprinted from the Journal of Alzheimer’s Disease, 44 (1), Chang KL, Pee HN, Tan WP, Dawe GS, Holmes E, Nicholson JK, Chan ECY, Ho PC, Metabolic profiling of CHO-AβPP695 cells revealed mitochondrial dysfunction prior to Amyloid-β pathology and potential therapeutic effects of both PPAR gamma and PPAR alpha agonisms for Alzheimer’s disease, 215-231, 2015, with permission from IOS Press.]
Reference
Chang KL, Pee HN, Tan WP, Dawe GS, Holmes E, Nicholson JK, Chan ECY, Ho PC. “Metabolic profiling of CHO-AβPP695 cells revealed mitochondrial dysfunction prior to Amyloid-β pathology and potential therapeutic effects of both PPAR gamma and PPAR alpha agonisms for Alzheimer’s disease”. Journal of Alzheimer’s Disease, 44 (1) (2015) 215.


