Optimising Drug Therapy of Prostate Cancer
Eric CHAN (Group Leader, Pharmacy) July 06, 2020NUS pharmaceutical scientists identified novel binding kinetics of the prostate cancer drug abiraterone to its pharmacological target. This allows optimisation of the therapeutic outcomes.
The dosage regimen of an anticancer drug is optimised based on its maximum tolerated dose and blood concentration. The latter is used as a surrogate of the drug concentration in the target tissue. This approach is scientifically reasonable assuming the drug is freely equilibrating between the blood and tissue compartments. However, for a drug that binds strongly to its pharmacological target, this assumption may not hold. Consequently, the dosing regimen that is designed based on this inaccurate assumption may be sub-optimal.
Currently, metastatic castration-resistant prostate cancer (mCRPC) patients are treated with 1000 mg of abiraterone acetate (prodrug) per day before food. The more soluble prodrug is converted within the gastrointestinal system to the active abiraterone prior to its systemic absorption. While the strong coordinate binding between abiraterone and its pharmacological target, cytochrome P450 17A1 enzyme (CYP17A1), has been previously established, its slow tight binding kinetics remained arcane. Prof Eric CHAN and his PhD student Eleanor CHEONG used in vitro and in silico studies together with mathematical modelling of clinical data to establish for the first time the extremely prolonged binding of abiraterone to CYP17A1. Consequently, the drug inhibits the enzyme for a period longer than the enzymatic life span. The research findings provide the impetus for re-evaluating the current dosing paradigm of abiraterone and potential efficacy at a reduced dose.
Recently, United States Food and Drug Administration (FDA) has further approved the use of abiraterone for the treatment of metastatic hormone-sensitive prostate cancer (mHSPC). The patients are expected to be prescribed with abiraterone over a longer period of time, which may cause dose-dependent adverse effects. Hence, an optimised dose would ensure that the therapy is efficacious, safe and cost-effective.
Prof Chan and Prof Edmund CHIONG (Head of Urology, National University Hospital) are currently planning a Phase 1 clinical trial to investigate the efficacy and safety of a reduced dosage of 500 mg abiraterone acetate once daily before food in both mCRPC and mHSPC patients.
“Understanding how abiraterone interacts with its pharmacological target at the atomic level kinetically helps us design a dosing regimen that preserves efficacy of abiraterone while mitigating its dose-dependent adverse effects for our patients”, says Prof Chan.
“Our Model-Informed Dose Optimisation (MIDO) platform, incorporating cross-disciplinary expertise, is a useful reference for scientists who are developing drugs that show prolonged target occupancy”, Prof Chan added.
This work is a collaboration with researchers from the National University Hospital, Singapore, Flinders University, Australia, A*STAR Bioinformatics Institute, Singapore, University of Chicago, USA and National Cancer Institute (NIH), USA.
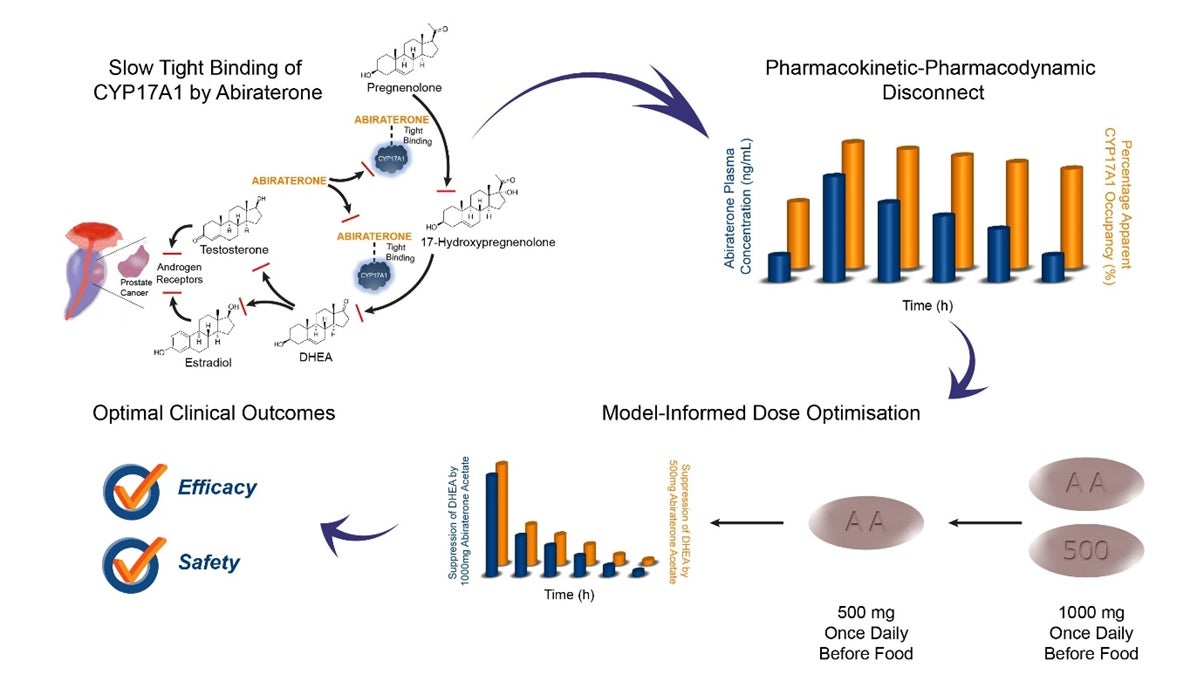
Figure shows the Model-Informed Dose Optimisation (MIDO) of abiraterone for the treatment of metastatic prostate cancers.
Reference
Cheong EJY; Nair PC; Neo RWY; Tu HT; Lin F; Chiong E; Esuvaranathan K; Fan H; Szmulewitz R; Peer CJ; Figg WD; Chai CLL; Miners JO; Chan ECY*, “Slow tight binding inhibition of CYP17A1 by abiraterone redefines its kinetic selectivity and dosing regimen” JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS DOI: https://doi.org/10.1124/jpet.120.265868.


