A new library of atomically thin two-dimensional materials
Kian Ping LOH (Group Leader, Chemistry) May 18, 2020NUS researchers have created a whole new library of atomically thin two-dimensional (2D) materials, christened “ic-2D”, to denote a class of materials based on self-intercalation of native atoms into the gap between the layers of crystals.
Atomically thin two-dimensional (2D) materials offer an excellent platform to explore a wide range of intriguing properties in confined 2D systems. However, compositional tuning of transition metal dichalcogenides to make new materials other than the standard binary or ternary compounds is challenging. In the past, theoreticians have tried to predict new properties based on combining atoms into a crystal structure where metal and chalcogen atoms sit in covalently bonded sites within the basic building block (unit cell). However, their theories did not address the situation when the same metal atom sits in between two unit cells (filling the van der Waals gap).
Now, research teams led by Prof Kian Ping LOH from the Department of Chemistry, Faculty of Science, NUS and collaborator Prof Stephen J. PENNYCOOK from the Department of Materials Science and Engineering, Faculty of Engineering, NUS, have synthesised and characterised for the first time, an atlas of wafer-scale atomically thin ic-2D materials based on inserting the same metal atoms between the van der Waals gap of transition metal dichalcogenides.
By performing growth under conditions where the metal atoms are in excess of the chalcogens (for example Sulphur (S), selenium (Se), Tellurium (Te)), over 10 different types of ic-2D materials have been experimentally discovered by the team. More excitingly, ferromagnetism was detected in some phases. In addition, high-throughput theoretical calculations show that the self-intercalation method is applicable to a large class of 2D layered materials. This means that there is a new library of ic-2D materials waiting to be discovered.
Prof Loh said, “This new method for engineering the composition of a broad class of transition metal dichalcogenides, offers a powerful approach to transform layered 2D materials into ultra-thin, covalently bonded ic-2D crystals with ferromagnetic properties. The main principle is the application of metal atoms with a high chemical potential to provide the driving force for intercalation during growth. This technique is expected to be compatible with most material growth methods.”
“ If we splice two layers of transition metal dichalcogenide a little apart, we can see the chalcogen sites having slots like an egg holder. Another layer of metal atoms can occupy the slots in the same way we can arrange eggs in the egg holder. This is the magic of ic-2D materials,” added Prof Pennycook.
Dr ZHAO Xiaoxu, the first author of the paper, discovered and atomically unveiled these novel materials using atomic-resolution scanning transmission electron microscopy, and found that intercalated metal atoms consistently occupy the octahedral vacancies inside the van der Waals gap resulting in distinct topographical patterns depending on the intercalation concentrations. Due to the unique toplogy, the ferromagnetism can be induced by the double exchange mechanism, triggered by the charge transfer from intercalated metal to pristine metal.
Prof Loh commented, “With versatility in composition control, we have shown that it is possible to tune, in one class of materials, properties that can vary from ferromagnetic to non-ferromagnetic, and spin-frustrated Kagome lattices. This discovery presents a rich landscape of ultra-thin 2D materials that await the further discovery of new properties.”
Next, the teams plan to incorporate this new library of materials into memory devices, for practical applications, and intercalate foreign atoms into the van der Waals gap and exploit novel functionalised ic-2D materials.
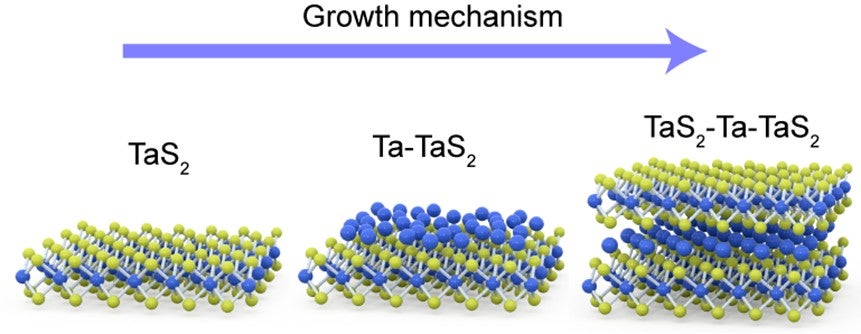
Researchers at NUS Chemistry, and Materials Science and Engineering have fabricated a whole new library of ic-2D materials by filling the van der Waals gap in (two-dimensional) 2D materials. Schematics showing the step-by-step growth of a typical Ta7S12 ic-2D material.
Reference
Zhao XX; Song P; Wang C; Riis-Jensen AC; Fu W; Deng Y; Wan D; Kang L; Ning S; Dan J; Venkatesan T; Liu Z; Zhou W; Thygesen K; Luo X*; Pennycook SJ*; Loh KP*, “Engineering Covalently Bonded 2D Layered Materials by Self-Intercalation” NATURE DOI: 10.1038/s41586-020-2241-9 Published: 2020.


